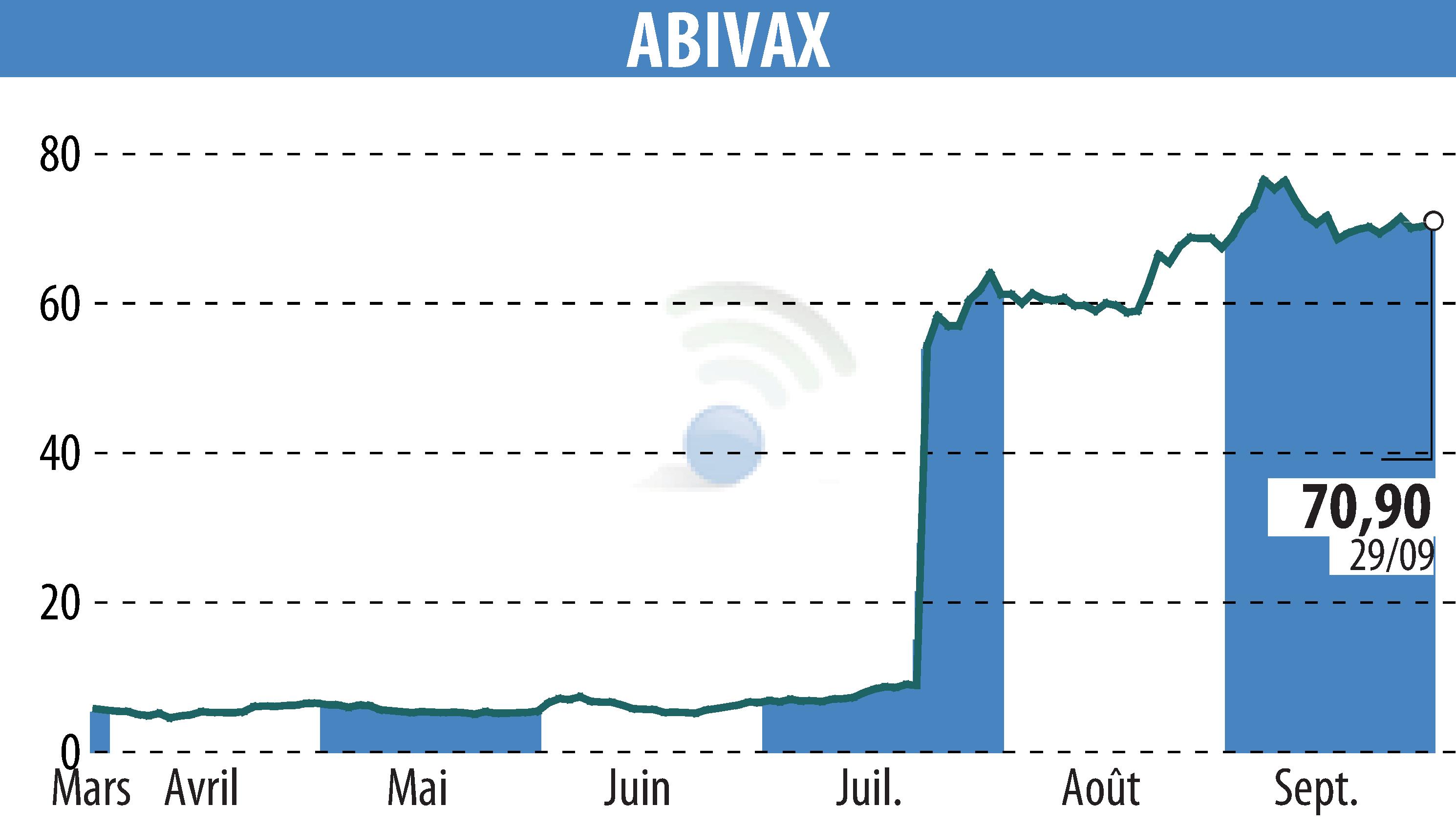
sur ABIVAX (EPA:ABVX)
Abivax Presents New Findings on Obefazimod at UEG 2025
Abivax has announced the acceptance of an additional late-breaking abstract for its lead drug candidate, obefazimod, in the treatment of ulcerative colitis. The findings, from their ABTECT Phase 3 induction trials, will be presented at the 2025 United European Gastroenterology (UEG) Meeting in Berlin. This presentation highlights significant results on the efficacy and safety of obefazimod during the 8-week trials, offering potential as a novel oral treatment for patients with moderately to severely active ulcerative colitis.
Dr. Fabio Cataldi, Abivax’s Chief Medical Officer, emphasized the statistical and clinical importance of these results in providing new treatment options for patients in need of therapeutic alternatives. Presentations by Dr. Bruce Sands and Dr. Silvio Danese will further explore the drug's effectiveness, including its impact on patients who had inadequate responses to previous therapies.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de ABIVAX
