sur ABSCIENCES (EPA:AB)
AB Science Gains Approval for Phase 3 ALS Masitinib Study Across Europe
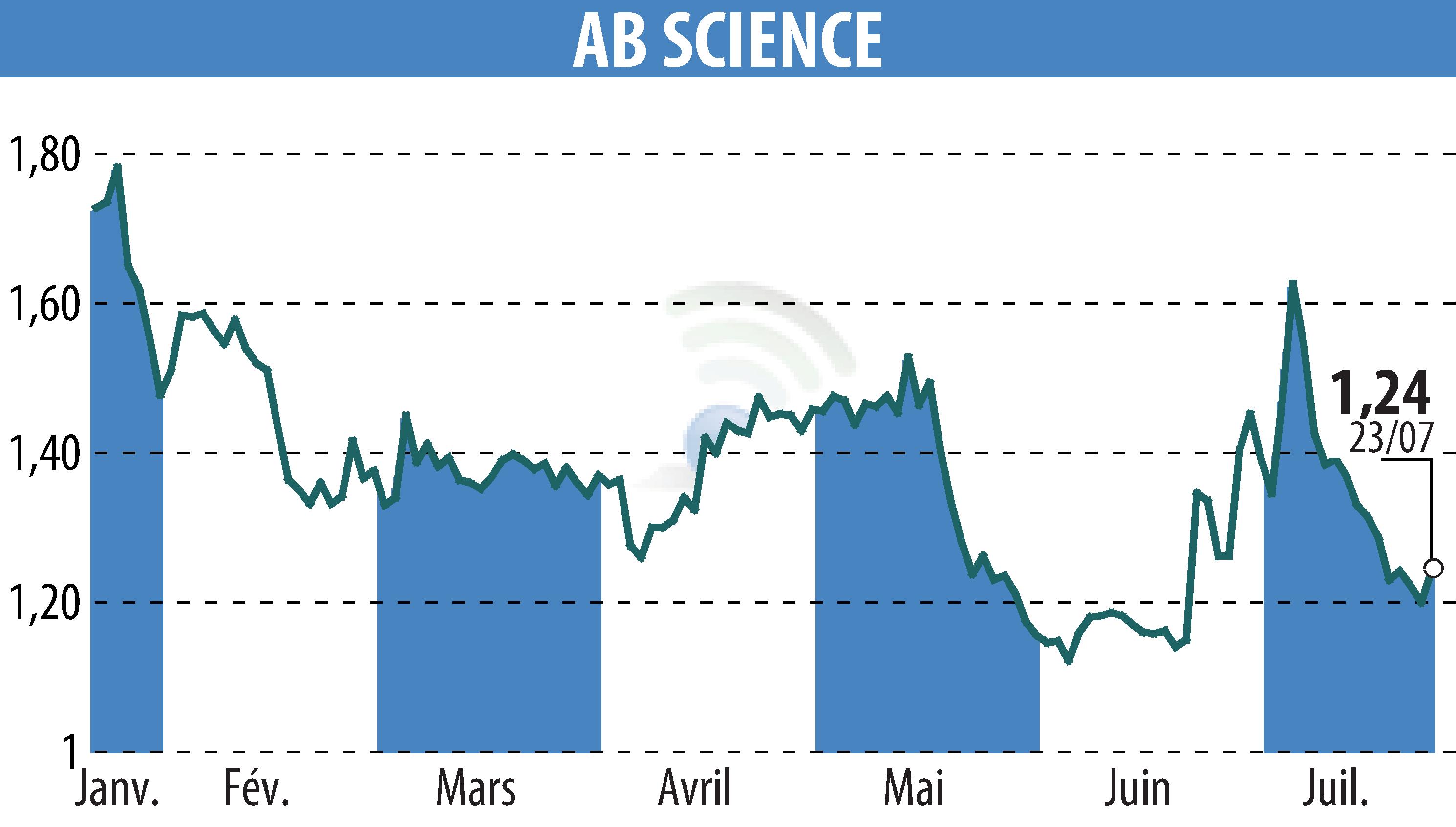
Paris-based AB Science has received authorization from various European nations, including Spain, Greece, and Slovenia, to begin the Phase 3 trial of masitinib in treating amyotrophic lateral sclerosis (ALS). This follows the European Medicines Agency's protocol validation and U.S. FDA authorization.
The Phase 3 trial, AB23005, aims to confirm masitinib's efficacy and safety in ALS patients. Conducted as a double-blind, placebo-controlled study, it will involve 408 patients with specific criteria regarding disease progression and functionality.
The study design, validated by European health authorities, targets patients with slower disease progression. Previous Phase 2B/3 results indicated potential survival benefits for this subgroup, with promising insights into masitinib's action on neurofilaments.
Masitinib, recognized for its immunomodulatory effects on mast cells and microglia, holds orphan drug status in Europe and the U.S., offering extended exclusivity in these markets.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de ABSCIENCES
