sur Aspire Biopharma, Inc. (NASDAQ:PWUP)
Aspire Biopharma Completes Dosing in Phase 1 Trial of High-Dose Aspirin
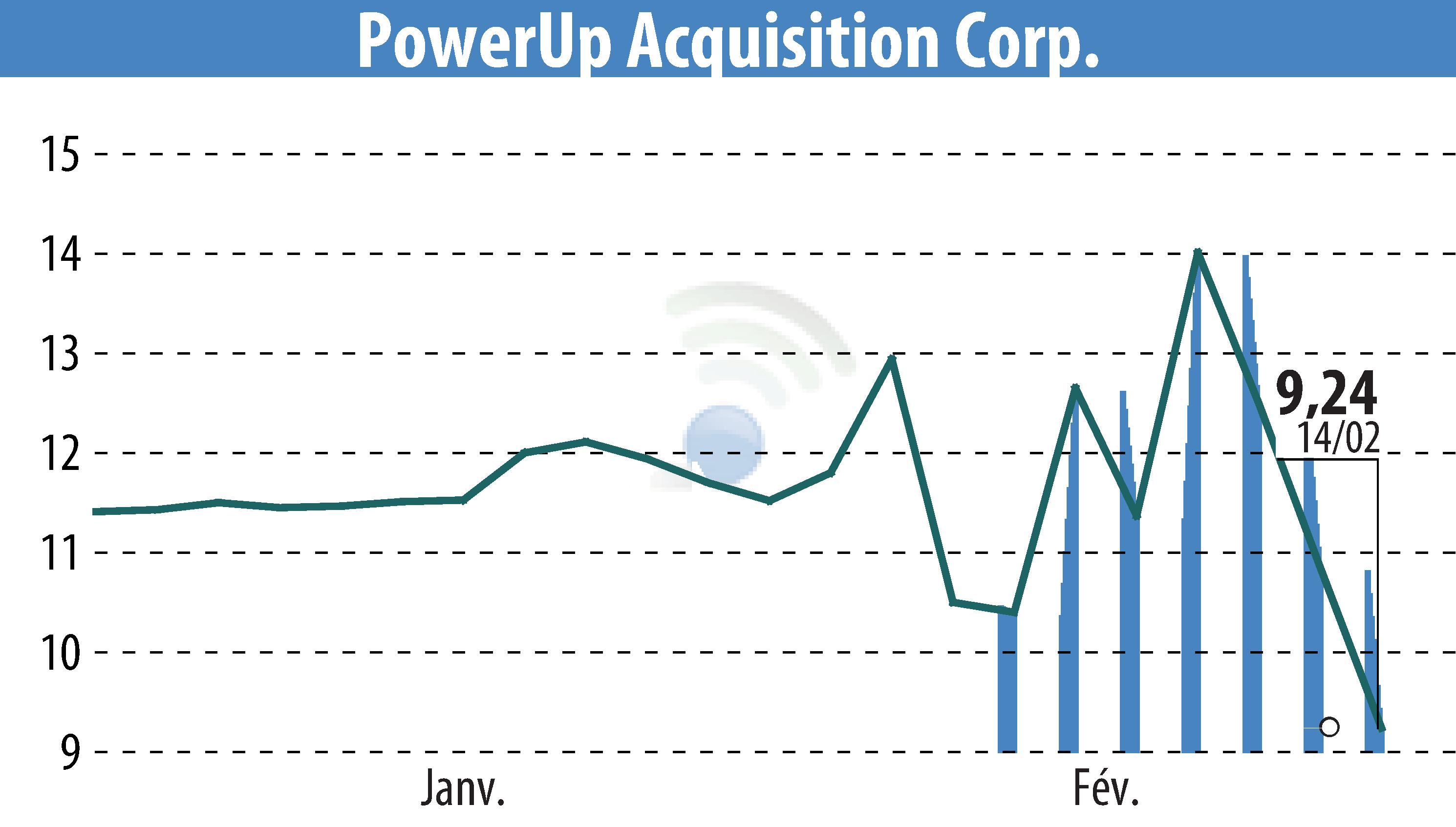
Aspire Biopharma Holdings, Inc. has announced the completion of patient dosing in its Phase 1 clinical trial for an oral transmucosal fast-acting high-dose aspirin formulation. The company expects topline results by mid-Q3 2025. CEO Michael Howe expressed gratitude towards participants and highlighted the milestone as a testament to their team's operational efficiency.
The trial compares the pharmacokinetic and pharmacodynamic characteristics of high-dose aspirin administered sublingually versus standard oral aspirin in healthy adults. Primary outcomes focus on plasma aspirin concentration and platelet aggregation metrics, key indicators of the formulation's rapid impact on cardiac events.
Aspire plans to seek FDA approval post-trial and integrate findings into its development strategy, with an eye on partnership opportunities. The study is crucial for their targeted oral delivery platform that enhances rapid drug absorption.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Aspire Biopharma, Inc.
