sur Biophytis (EPA:ALBPS)
Biophytis Initiates Phase 2 OBA Clinical Trial for Obesity Treatment
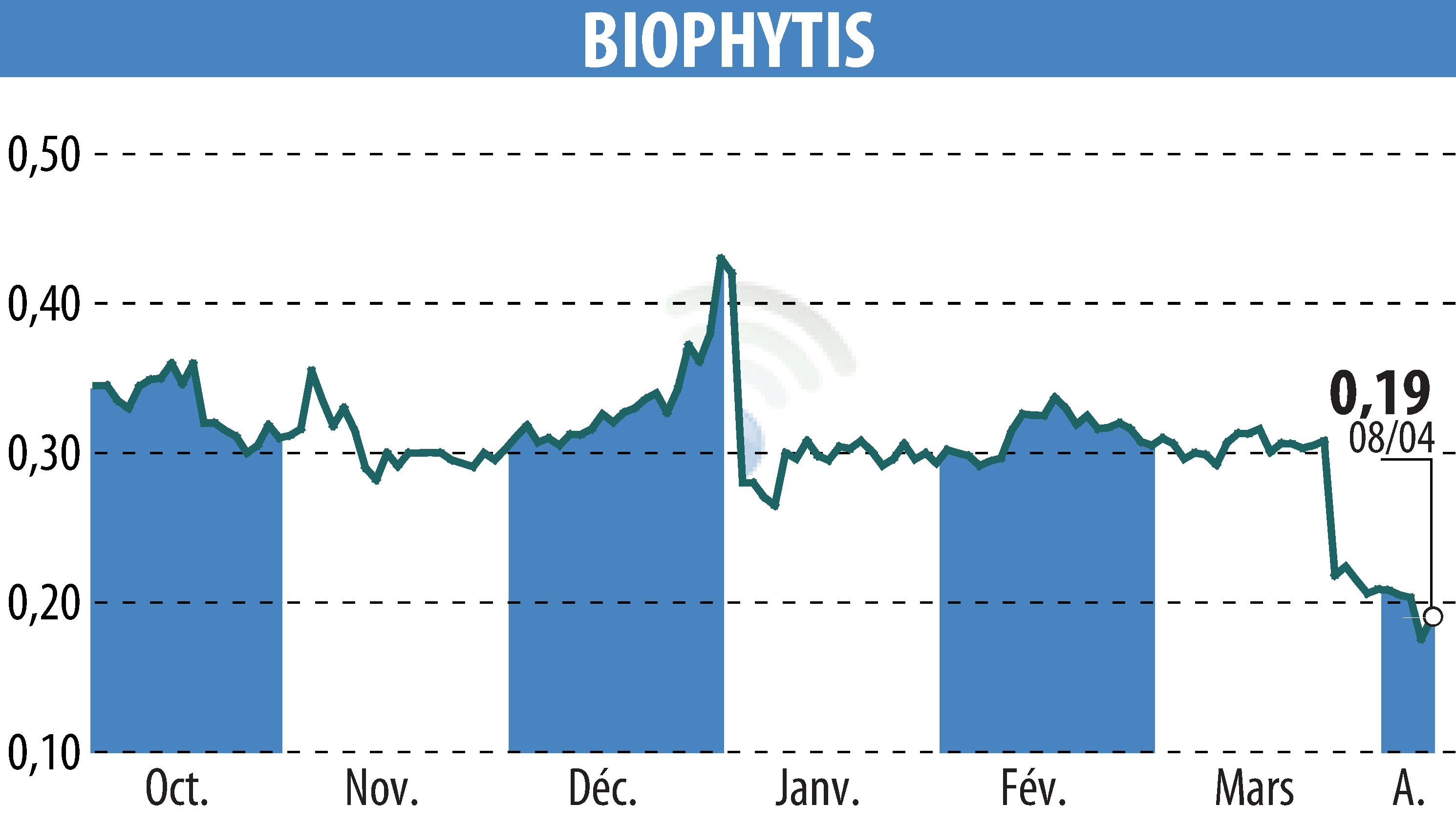
Biophytis SA, a biotechnology firm specializing in treatments for age-related diseases, has confirmed the launch of its Phase 2 OBA clinical trial targeting obesity. The study aims to evaluate the efficacy and safety of their drug candidate, BIO101, in countering muscle strength loss induced by GLP-1 agonists in obese patients. The trial is scheduled to commence in early 2025.
Following a capital increase of €2.6 million and promising preclinical outcomes, Biophytis is poised to advance its clinical studies. The U.S. FDA's Investigational New Drug clearance in July 2024 marked a significant step for the OBA program. The initiative leverages the expertise of Professor Marc-André Cornier in the obesity domain.
The company is actively seeking a pharmaceutical partner to facilitate the subsequent clinical development and market launch in North America.
R. P.
Copyright © 2025 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Biophytis
