sur CARMAT (EPA:ALCAR)
CARMAT Achieves Strong Q1 2025 Results
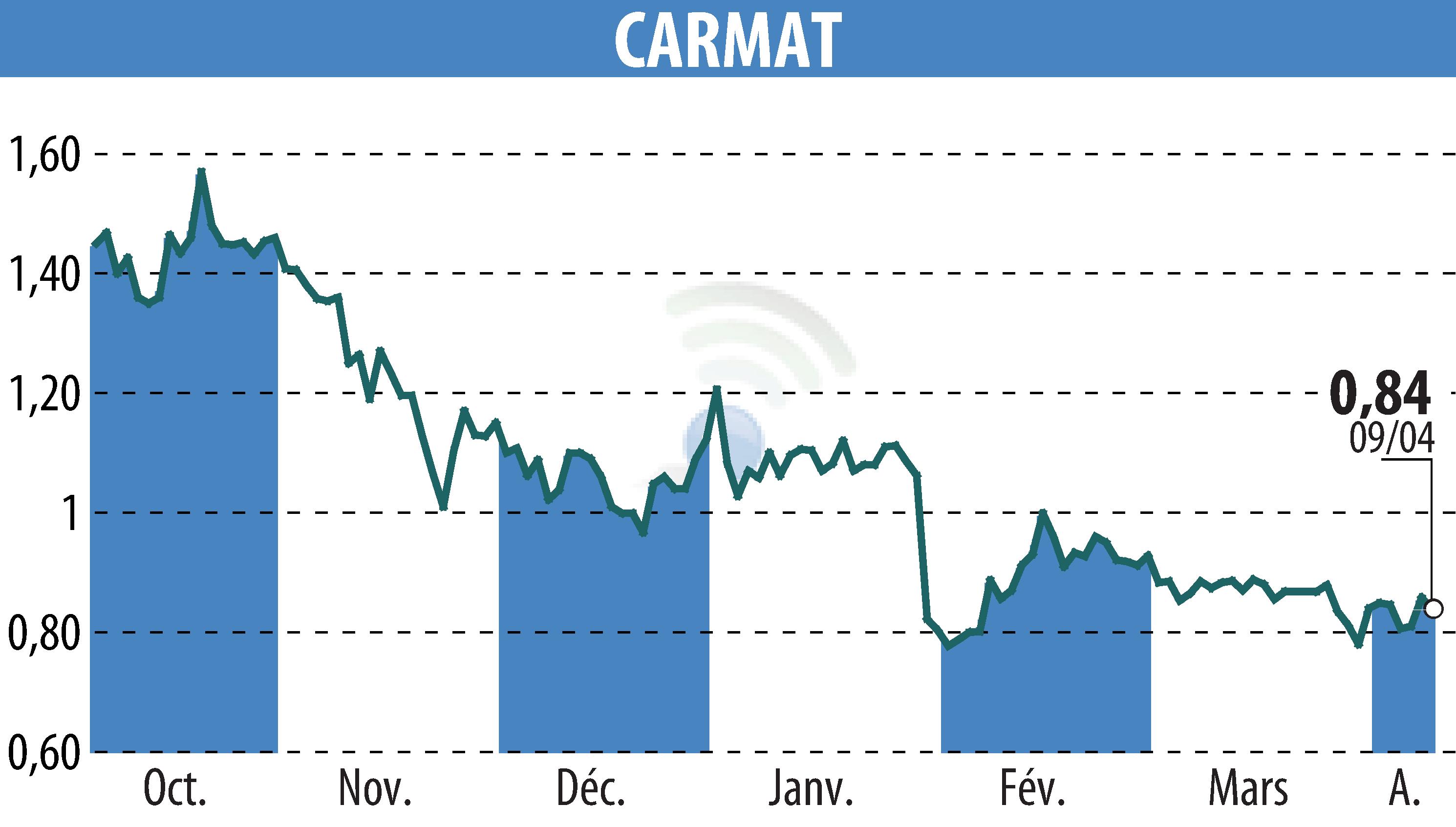
CARMAT reported Q1 2025 sales of €2.4 million, doubling from the same period last year. The company executed 16 Aeson® artificial heart implants across France, Germany, and Italy, highlighting increased adoption.
The EFICAS clinical trial has nearly completed recruitment with 94% enrolled, moving towards its conclusion. Outcomes from this study are crucial, as they are expected to drive both national and international adoption of Aeson®.
CARMAT is finalizing discussions with the FDA to restart the Early Feasibility Study in the U.S., a significant step for accessing this major market. Additionally, the company's financial strategy includes recent fundraising efforts to maintain liquidity through mid-2025.
The newly appointed Medical Director, Professor Christian Latrémouille, succeeds Dr. Piet Jansen, marking a transition aimed at further anchoring Aeson® in clinical practice.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de CARMAT
