sur DBV TECHNOLOGIES (EPA:DBV)
DBV Technologies Reports Positive Phase 3 Results for VIASKIN Peanut Patch
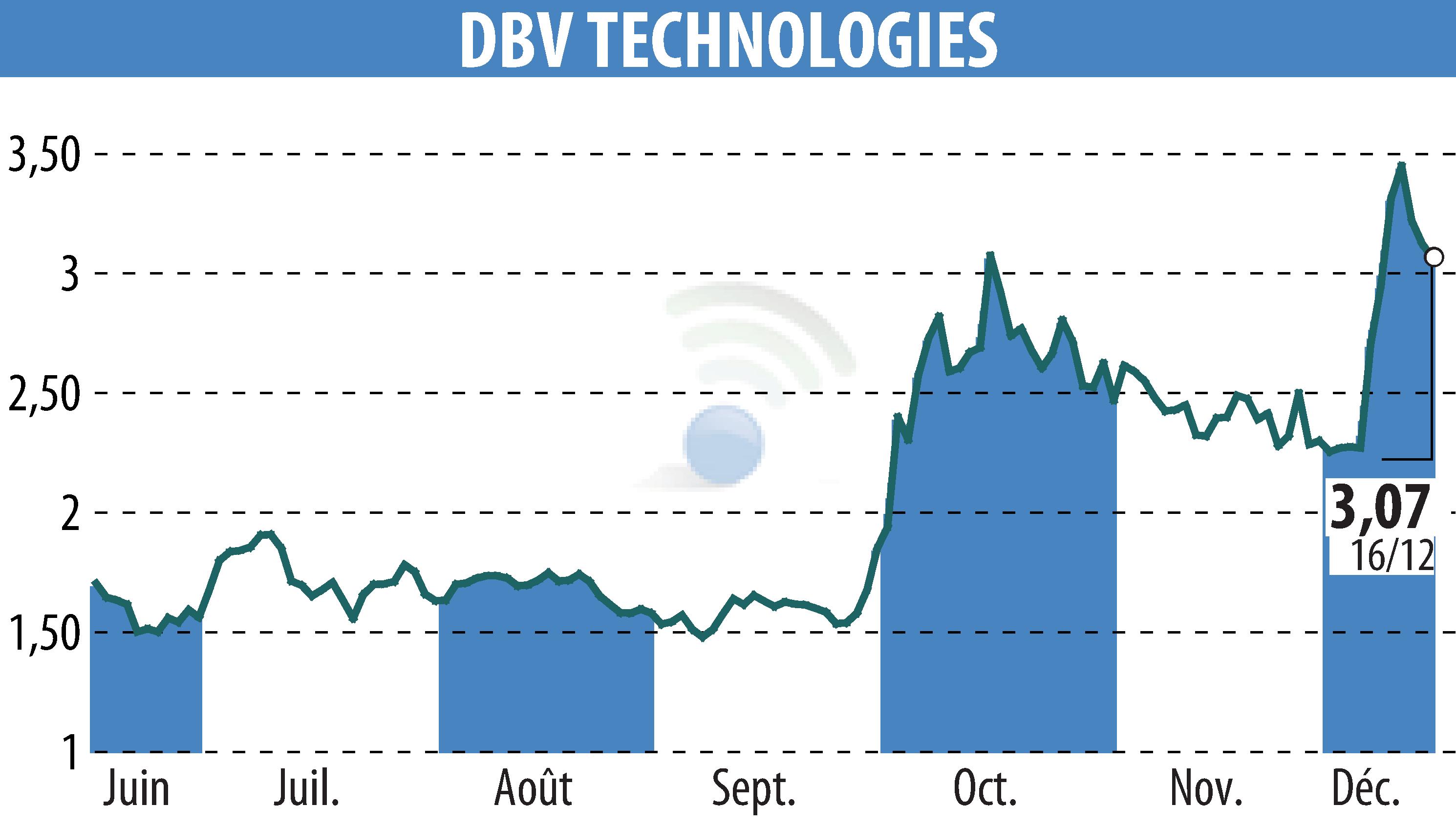
DBV Technologies has announced favorable results from its Phase 3 VITESSE trial, evaluating the VIASKIN Peanut patch in children aged 4-7 with peanut allergies. The study met its primary endpoint, with a significant 46.6% response rate in the treatment group compared to 14.8% in the placebo group. This marks a 24.5% difference, surpassing the set threshold of 15%.
The trial involved 654 children, and adverse events were mostly mild to moderate skin reactions. Notably, no serious treatment-related events were reported. The company plans to submit a Biologics License Application (BLA) in the US by mid-2026, potentially leading to the VIASKIN Peanut patch becoming a new option for managing peanut allergy in young children.
DBV will also accelerate its warrant exercise period following these positive results, potentially impacting their upcoming financial strategies.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de DBV TECHNOLOGIES
