sur EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Medical Reports Q3 2025 Financial Results
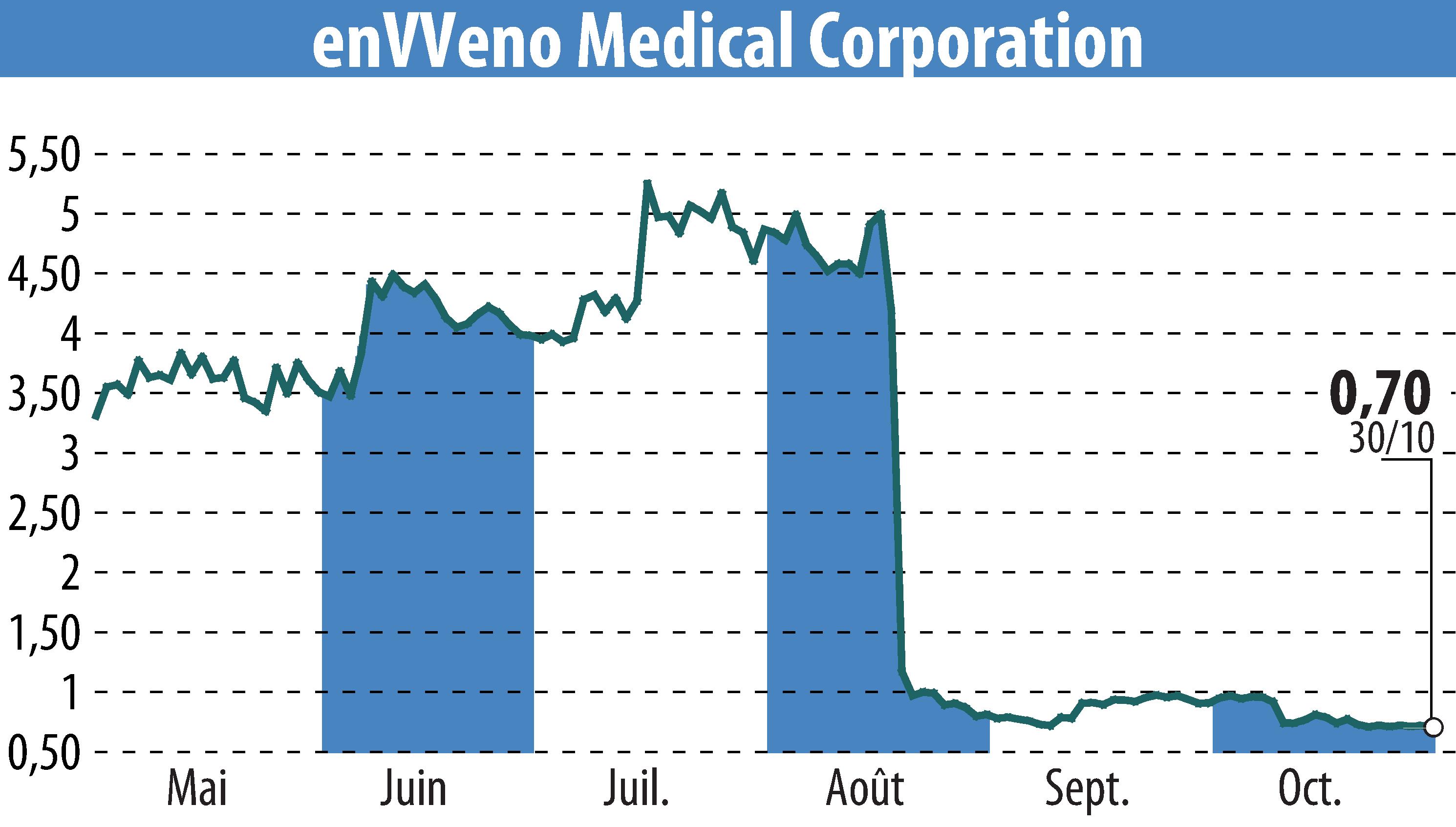
enVVeno Medical Corporation has revealed its third-quarter 2025 financial results, maintaining a cash reserve of $31.0 million. This reserve is expected to support the company through the second quarter of 2027, excluding the costs associated with the VenoValve commercialization and the enVVe IDE study. The company's cash burn rate for the quarter was $4.2 million, aligning with projections of $4-5 million per quarter.
The company reported a net loss of $4.5 million, a reduction from the $5.6 million loss reported in the third quarter of 2024. This improvement is attributed to a decrease in operating expenses by $1.3 million. enVVeno is engaged with the FDA after filing for a supervisory appeal following a not-approvable letter for the VenoValve. A resolution is anticipated by the end of 2025.
The company continues to advance with the enVVe program, having completed necessary preclinical tests and is preparing for an IDE filing contingent on outcomes from the VenoValve appeal.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de EnVVeno Medical Corporation
