sur ESSILOR (EPA:EL)
EssilorLuxottica's Essilor Stellest Lens Gains FDA Approval
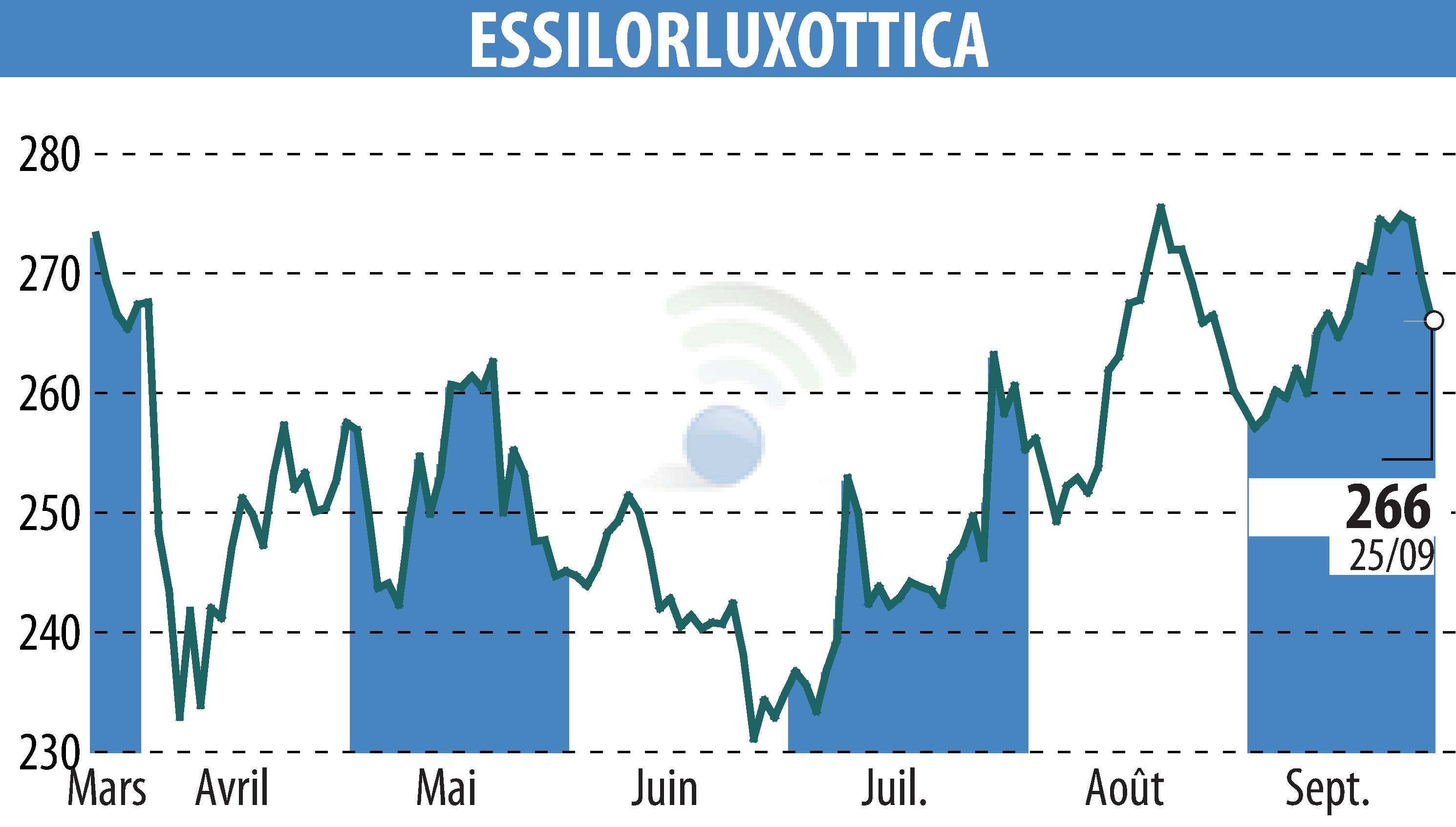
EssilorLuxottica announced that its Essilor Stellest lens has received market authorization from the U.S. FDA, using the De Novo pathway. This makes it the first FDA-approved spectacle lens clinically proven to slow myopia progression in children. Clinical trials demonstrate a 71% reduction in myopia progression over two years.
Francesco Milleri, CEO of EssilorLuxottica, highlighted the lens as a significant innovation in vision care. The company has been a leader in myopia research for over 40 years, aiming to deliver solutions to address the growing myopia epidemic affecting children globally. The lens will soon be available to U.S. eyecare professionals.
With myopia affecting 1 in 4 children in North America, Essilor Stellest offers a critical solution in mitigating its progression and improving eye health outcomes.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de ESSILOR
