sur GENSIGHT BIOLOGICS S.A. (EPA:SIGHT)
GenSight Biologics Begins REVISE Study for GS010 Therapy
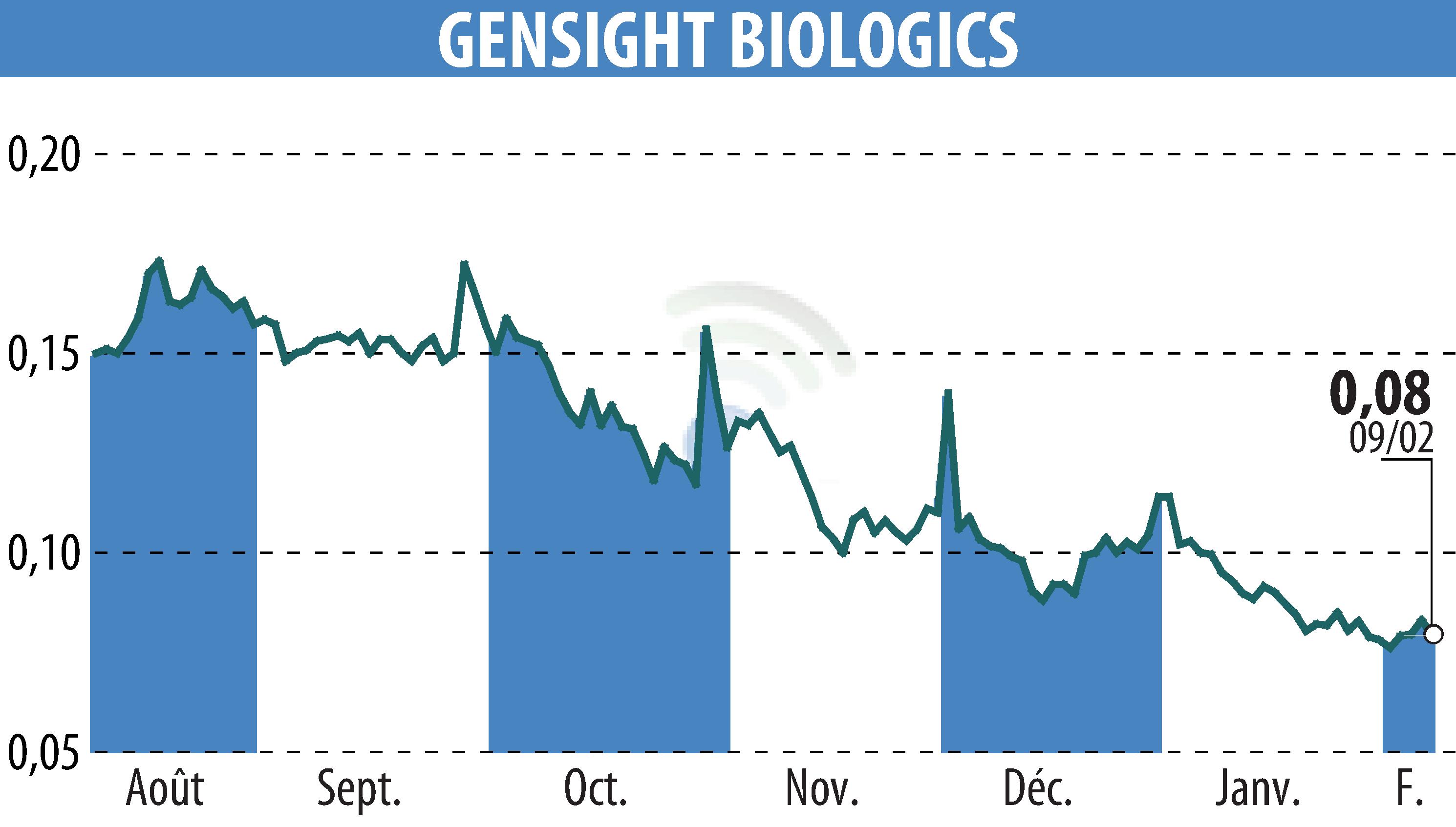
The 15-20 National Hospital in Paris and biopharma company GenSight Biologics have initiated the treatment of the first patient in the GS010/LUMEVOQ® REVISE study. Approved by France's ANSM, this dose-ranging study will include 14 patients. The study advances ongoing efforts to develop transformative treatments for rare diseases like Leber Hereditary Optic Neuropathy (LHON).
Currently, the 15-20 National Hospital is the sole European facility conducting clinical studies with GS010, a gene therapy candidate aimed at treating LHON caused by ND4 mitochondrial mutations. The study's initiation highlights the hospital's support for significant scientific advancements. The French ANSM authorized both the REVISE study and an early access program in December 2025.
GS010 has not yet received marketing approval. However, its clinical development progresses, underscoring the urgency to address unmet medical needs in LHON patients.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de GENSIGHT BIOLOGICS S.A.
