sur GENSIGHT BIOLOGICS S.A. (EPA:SIGHT)
GenSight Biologics: Green light for compassionate treatment in the United States
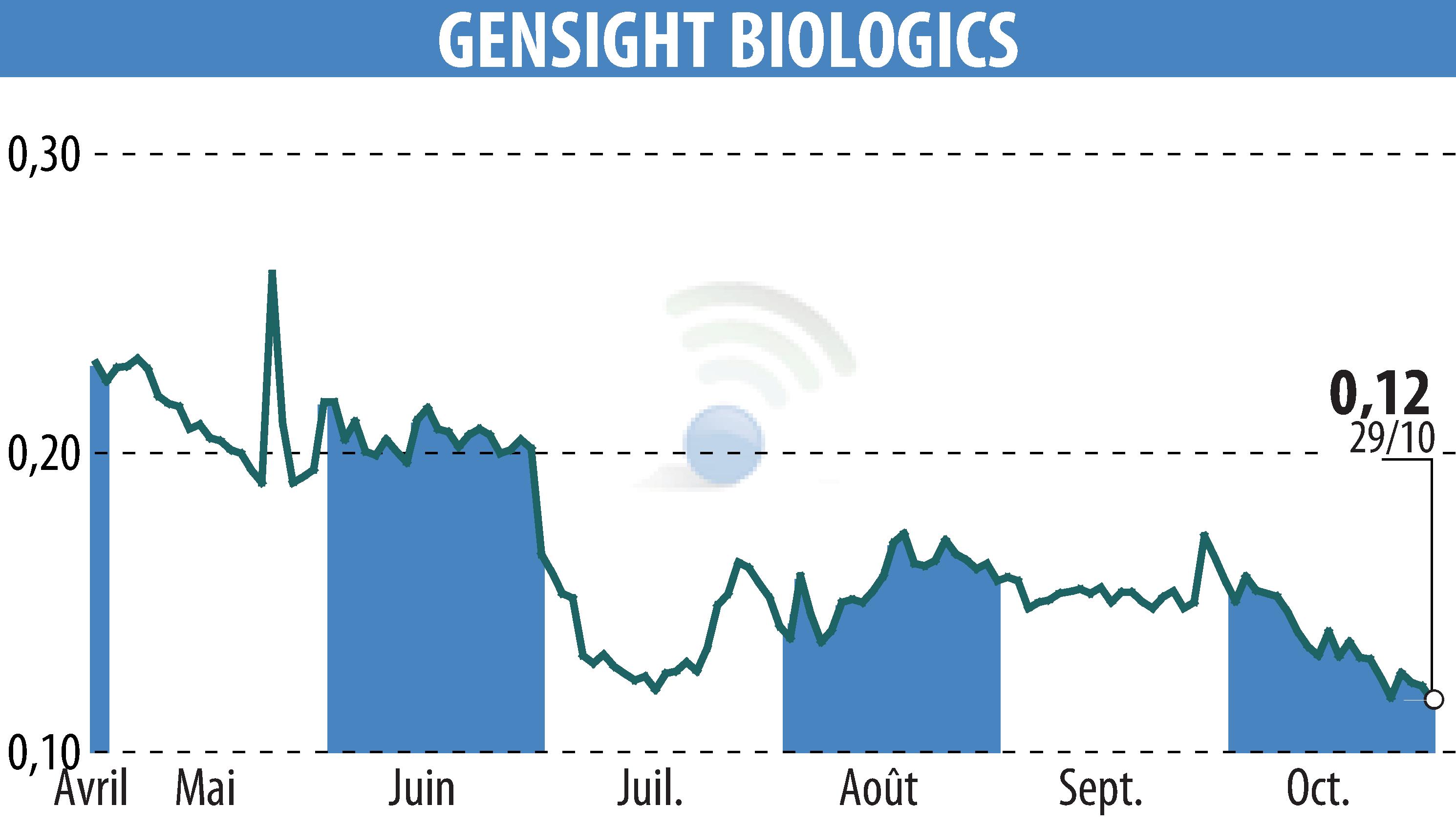
GenSight Biologics has received regulatory approval to administer its innovative gene therapy, GS010/LUMEVOQ®, to a patient in the United States under a compassionate access program. Following approval from the Food and Drug Administration (FDA) and the Institutional Review Board, the treatment is scheduled to begin in November 2025 at the University of Pittsburgh.
GS010/LUMEVOQ® targets Leber hereditary optic neuropathy, a rare disease causing blindness due to a mutation in the mitochondrial ND4 gene. While the product is in phase III clinical development, it still does not have commercial status in any country.
At the same time, the company is continuing its efforts to transfer its manufacturing technologies to its partner, Catalent, and is exploring international licensing opportunities, while negotiating compassionate access in France with the ANSM.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de GENSIGHT BIOLOGICS S.A.
