sur GENSIGHT BIOLOGICS S.A. (EPA:SIGHT)
GenSight Biologics Receives Compassionate Use Authorization for GS010/LUMEVOQ® in France
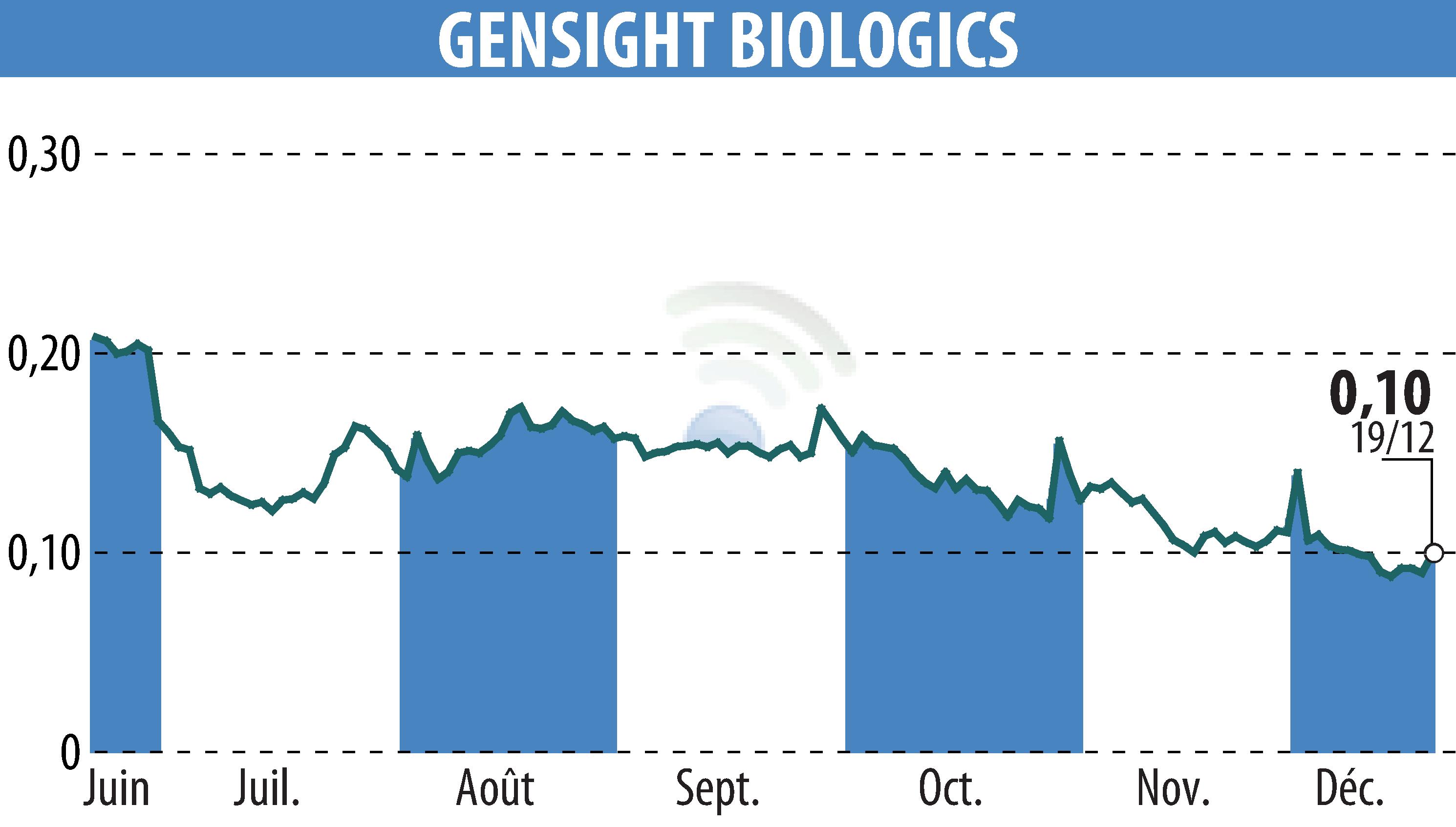
GenSight Biologics, a clinical-stage biopharma company, has announced the approval of a compassionate use authorization for their gene therapy GS010/LUMEVOQ® by the French medicines agency ANSM. This authorization allows early access for patients suffering from ND4-LHON, a rare genetic disease affecting vision.
The compassionate use program in France is designed for patients with serious, rare, or disabling diseases, providing them with treatments that lack marketing authorization, addressing unmet medical needs. The program requires a favorable benefit-risk assessment and is initiated by healthcare professionals who submit individual requests. Eligible patients must meet specific criteria related to the duration of their vision loss.
Leber Hereditary Optic Neuropathy (LHON) is a rare genetic condition causing retinal degeneration and vision loss. The ND4 mutation leads to the most severe outcomes among its types. GS010/LUMEVOQ® leverages proprietary mitochondrial targeting technology for treatment.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de GENSIGHT BIOLOGICS S.A.
