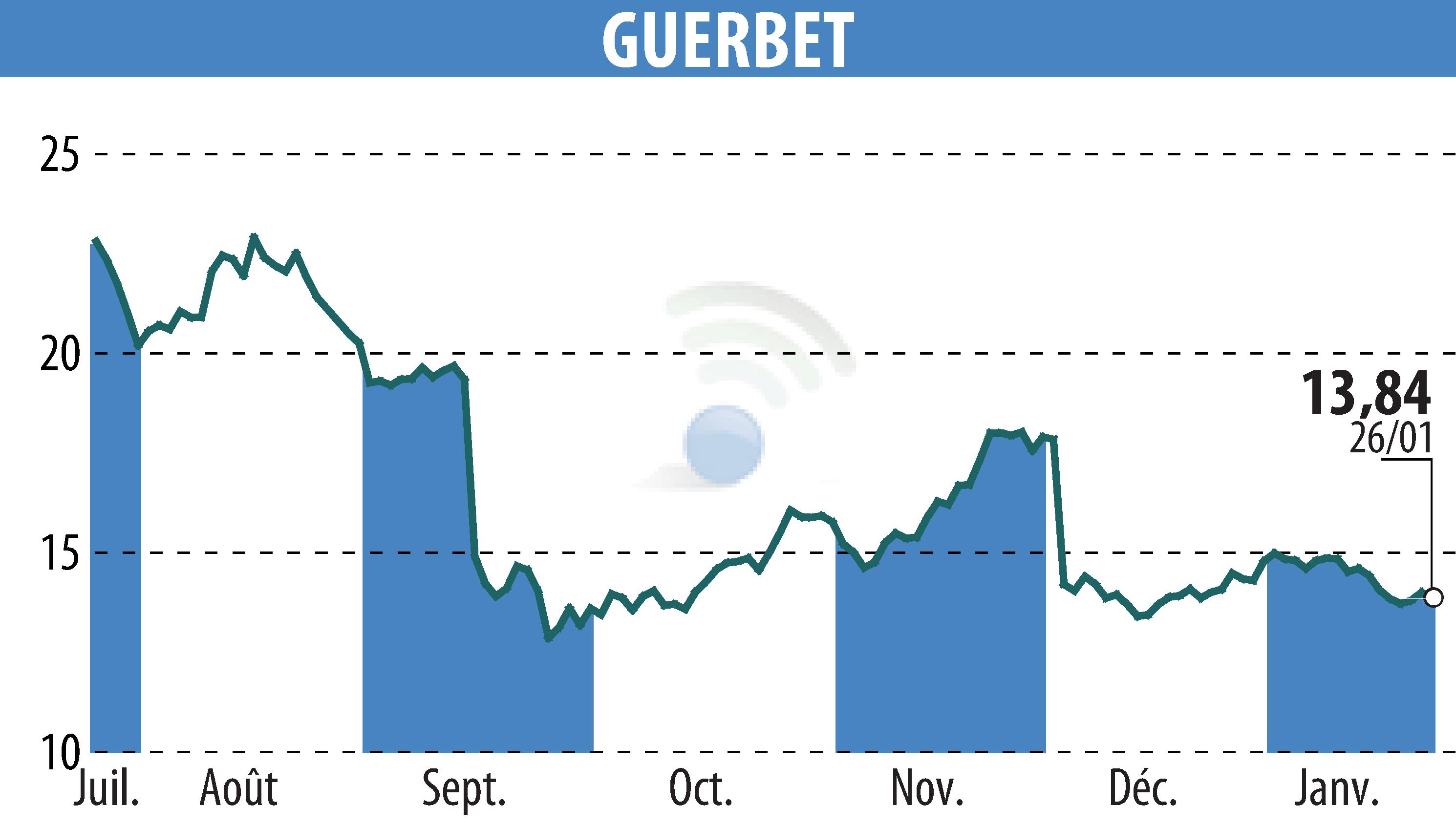
sur GUERBET (EPA:GBT)
European Approval for Guerbet's Elucirem™ Use in Children from Birth
Guerbet, a leader in medical imaging solutions, has received European Commission approval for a new indication of its gadolinium-based contrast agent, Elucirem™ (Gadopiclenol), now usable in children from birth. This approval marks a significant advancement as Elucirem™ is noted for its high relaxivity, offering improved image quality at half the conventional gadolinium dose.
First approved in the EU in December 2023, Elucirem™ is already used in adults for contrast-enhanced MRIs of the central nervous system and major organs. The lower dose requirement is particularly valuable for pediatric patients, reducing cumulative gadolinium exposure.
Valérie Brissart, Guerbet's SVP, underlined the commitment to medical innovation and patient safety, emphasizing the reduction in gadolinium exposure during serial MRI examinations. Dr. Emilio J Inarejos Clemente from Hospital Sant Joan de Déu noted the benefit of high-quality imaging with minimized gadolinium exposure for vulnerable pediatric patients.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de GUERBET
