sur Heidelberg Pharma AG (ETR:HPHA)
FDA Requests Additional Data from Telix Pharmaceuticals, Delaying Payment to Heidelberg Pharma
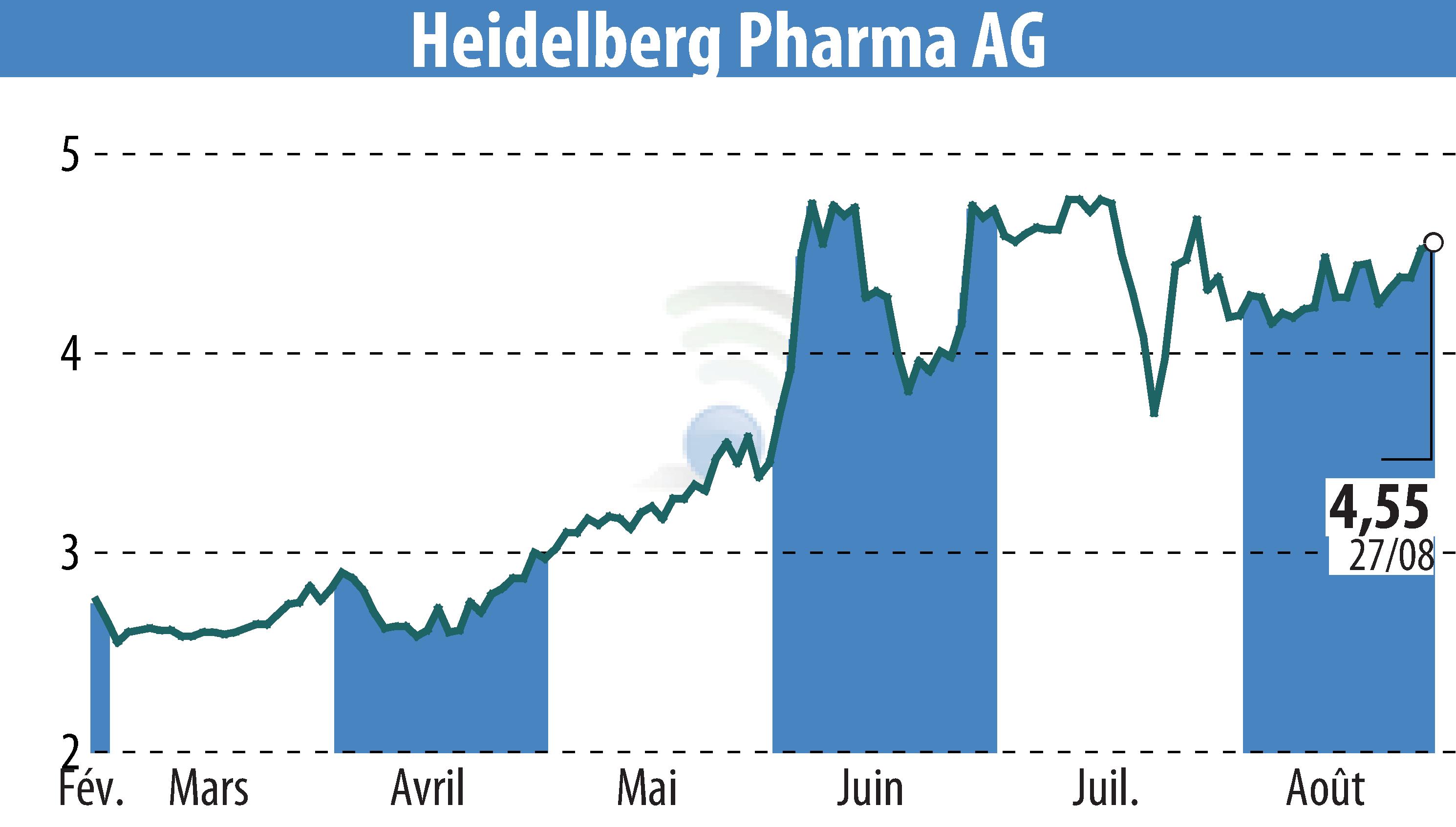
Heidelberg Pharma AG's partner, Telix Pharmaceuticals, encountered a regulatory setback concerning its imaging agent, TLX250-CDx. The FDA has raised concerns over the Chemistry, Manufacturing, and Controls (CMC) package, necessitating further data. This has resulted in a delay in a planned USD 70 million payment from HealthCare Royalty to Heidelberg Pharma.
Telix had submitted a Biologic License Application (BLA) for TLX250-CDx, which received a Priority Review. Despite initial acceptance, a Complete Response Letter was issued due to identified deficiencies. Telix is tasked with proving comparability between the drug used in the ZIRCON Phase 3 trial and the intended commercial product. Two third-party partners also need to address deficiencies.
This development affects Heidelberg Pharma's financial timeline, with cash reserves expected to last until the first quarter of 2026.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Heidelberg Pharma AG
