sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Advances Development with FDA Meeting on Crofelemer for MVID Treatment
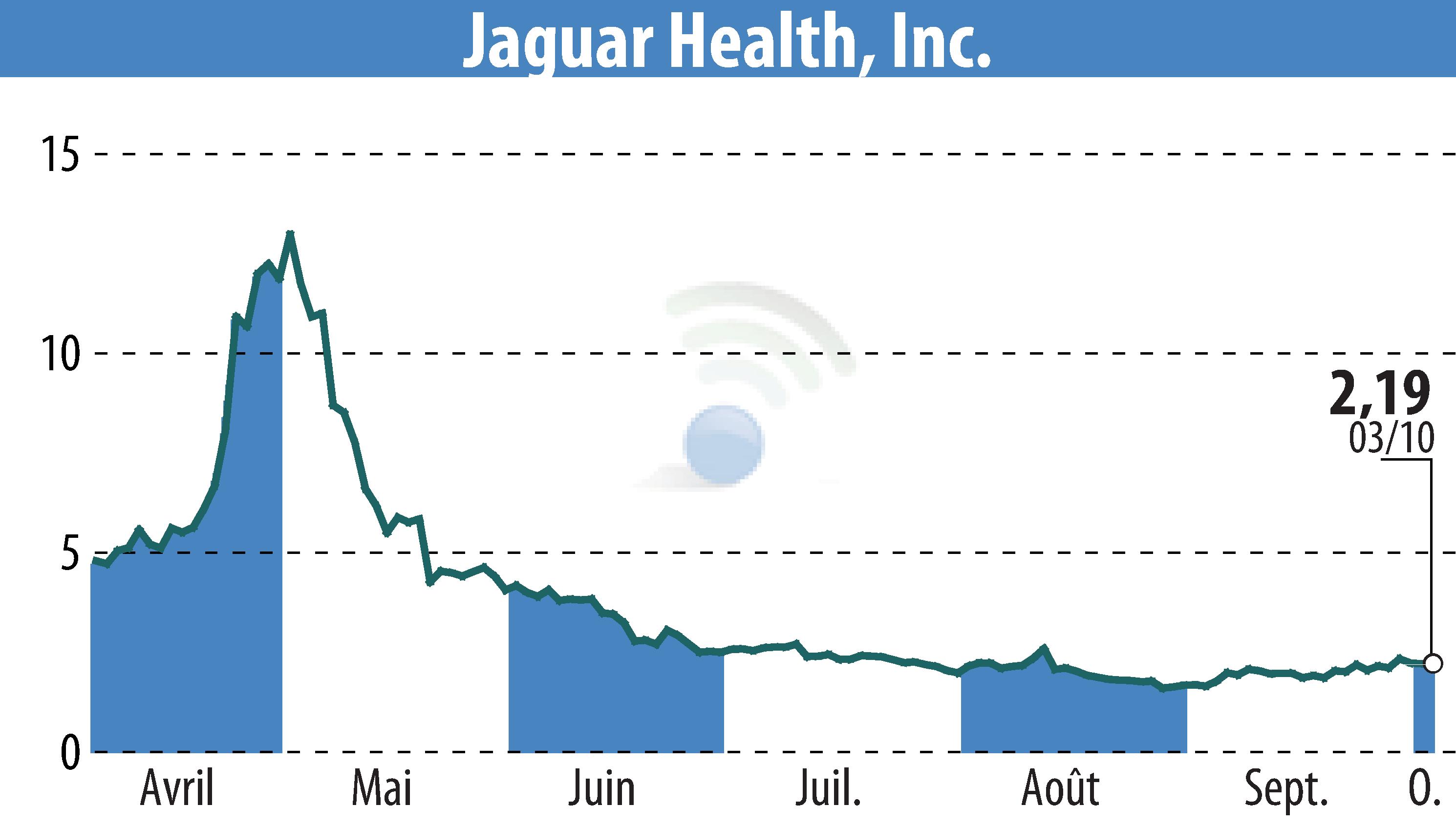
Jaguar Health, Inc., alongside its subsidiary Napo Pharmaceuticals, has completed a Type C meeting with the FDA to discuss the progression of its crofelemer trial intended for microvillus inclusion disease (MVID), a rare pediatric condition. MVID affects approximately 100-200 patients worldwide and currently lacks approved treatments. Notable participants in the meeting included key opinion leaders and scientific advisors.
Preliminary trial results in the UAE show a 37% reduction in parenteral support, indicating promising progress. Jaguar is keen to amend the trial based on FDA feedback to align with regulatory expectations. Crofelemer has received orphan drug status from both the FDA and EMA, supporting Jaguar's push for global availability.
The company plans to further liaise with regulatory bodies in the EU and MENA regions. An abstract of the UAE trial has been accepted for presentation at the NASPGHAN Annual Meeting in Chicago this November.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
