sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Launches Study for Canalevia-CA1 Drug in Dogs
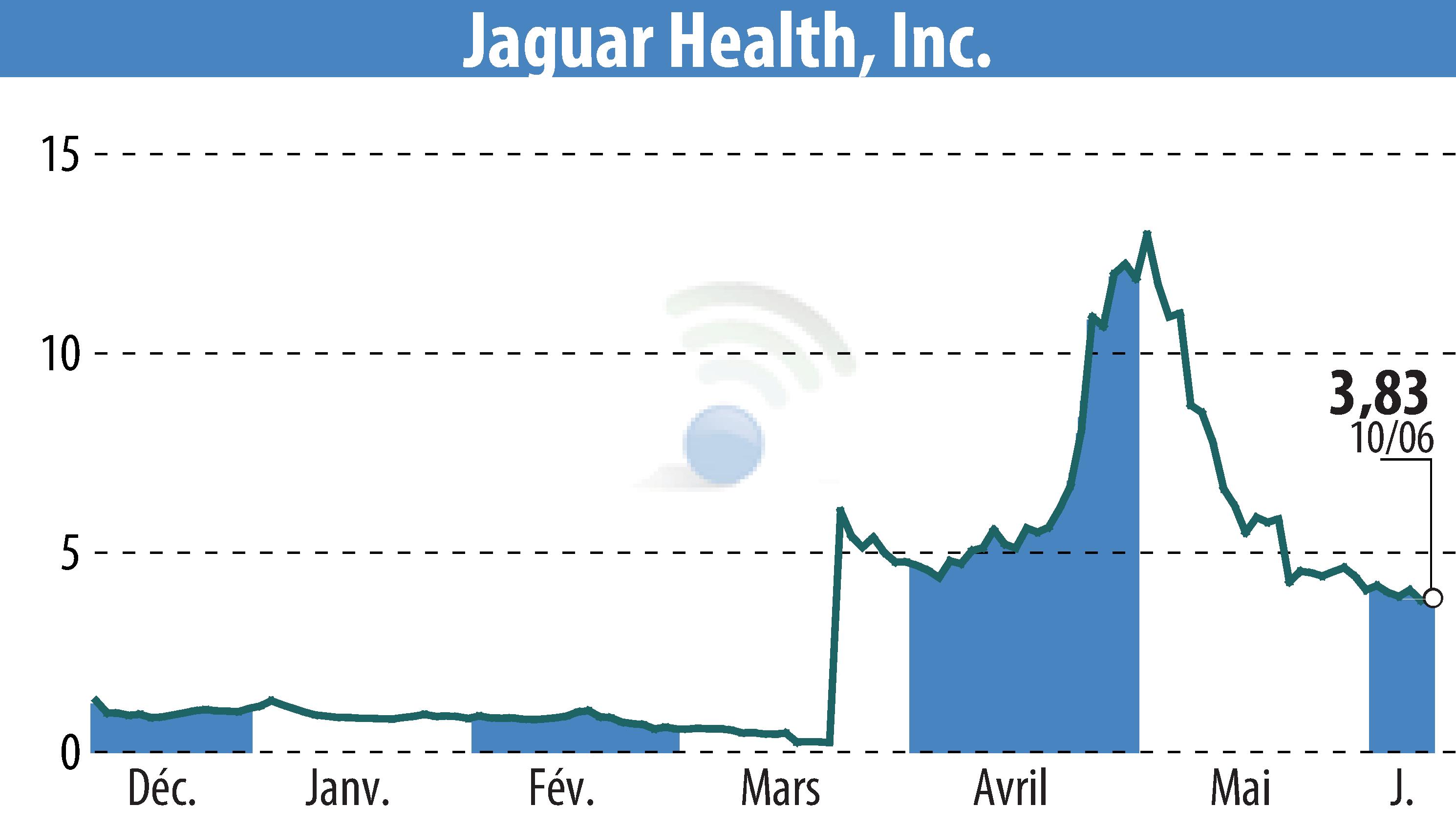
Jaguar Health, Inc. has initiated a study site for its Canalevia-CA1 drug, conditionally approved by the FDA for treating chemotherapy-induced diarrhea (CID) in dogs. The company's main objectives include securing full approval for this use and extending its application to general, non-infectious diarrhea in dogs.
Diarrhea remains a leading reason for veterinary consultations, and currently, no FDA-approved medications are available for general diarrhea in canines. In response, Jaguar is in discussions with partners to develop and market crofelemer, the active component of Canalevia-CA1, addressing this market gap both in the U.S. and internationally.
The ongoing study seeks to gather evidence supporting the clinical efficacy of Canalevia-CA1 in CID treatment. This data collection is crucial for achieving FDA's full approval, a significant step for Jaguar's business strategy in 2025.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
