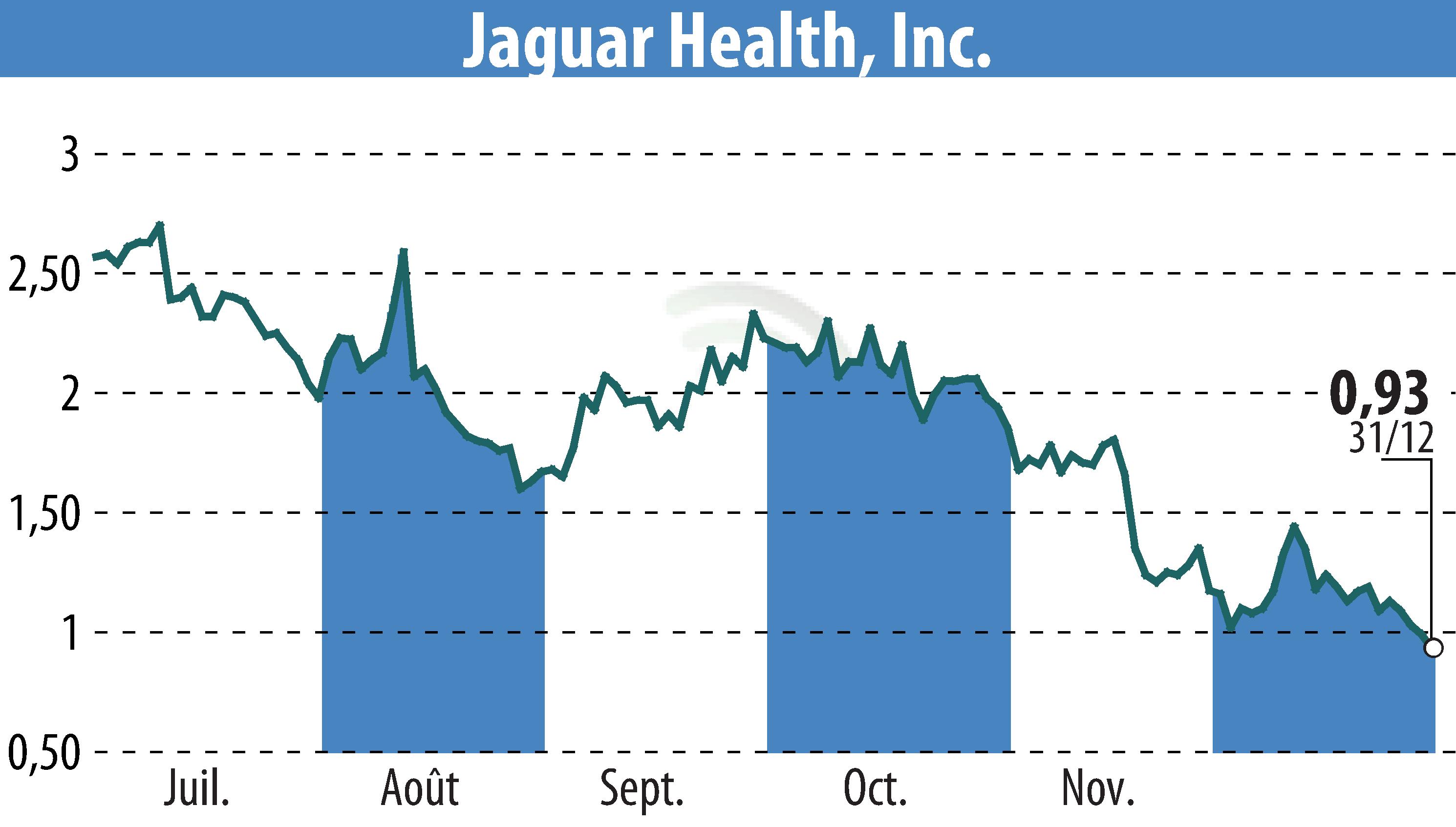
sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Secures FDA Grant for Canalevia-CA1 Studies
Jaguar Health, Inc. announced on January 2, 2026, the receipt of a $240,000 grant from the U.S. Food and Drug Administration's Center for Veterinary Medicine. This funding supports ongoing studies of Canalevia-CA1, a conditionally approved treatment for chemotherapy-induced diarrhea (CID) in dogs. The FDA has recently renewed this conditional approval for a fifth year while requiring a comprehensive efficacy study.
Canalevia-CA1 addresses a crucial need, given that CID is a prevalent issue in dogs undergoing cancer treatment. With about 90 million dogs in the U.S., Jaguar estimates over 11 million suffer from general diarrhea annually. CID is a significant complication affecting canine cancer patients that can hinder effective treatment regimens.
Notably, Canalevia is not an antibiotic, countering the trend of antibiotic resistance by avoiding unnecessary use. This highlights the importance of non-antibiotic therapies in combating diarrhea. The absence of an FDA-approved drug for general diarrhea in dogs underscores a market gap that Jaguar seeks to fill.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
