sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Seeks EMA Approval for Canalevia to Treat Dog Diarrhea
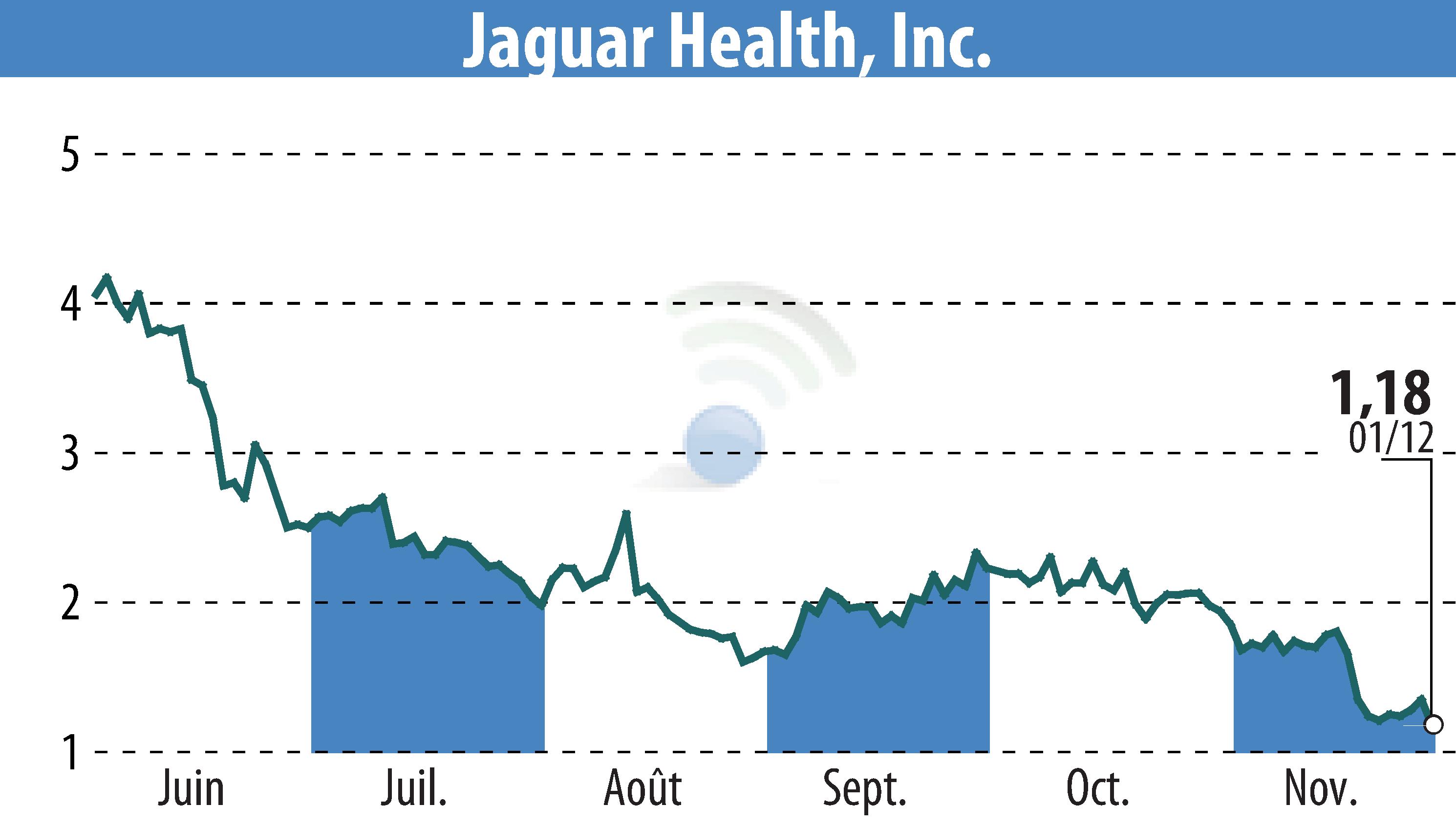
Jaguar Health, Inc. has taken a critical step towards expanding its canine gastrointestinal treatment, Canalevia, in the European Union. The company has submitted a request to the European Medicines Agency (EMA) for scientific advice concerning the approval pathway for Canalevia, aimed at treating general diarrhea in dogs. This submission comes on the heels of a study involving 200 dogs, which initially did not meet its primary endpoint. However, a revised analysis indicated better outcomes for dogs treated with Canalevia compared to placebo.
Canalevia, conditionally approved in the U.S. for chemotherapy-induced diarrhea, represents a novel non-antibiotic approach to treating one of the most common veterinary issues. Jaguar aims to secure a partner for global development and commercialization, especially given the lack of FDA-approved options for general canine diarrhea.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
