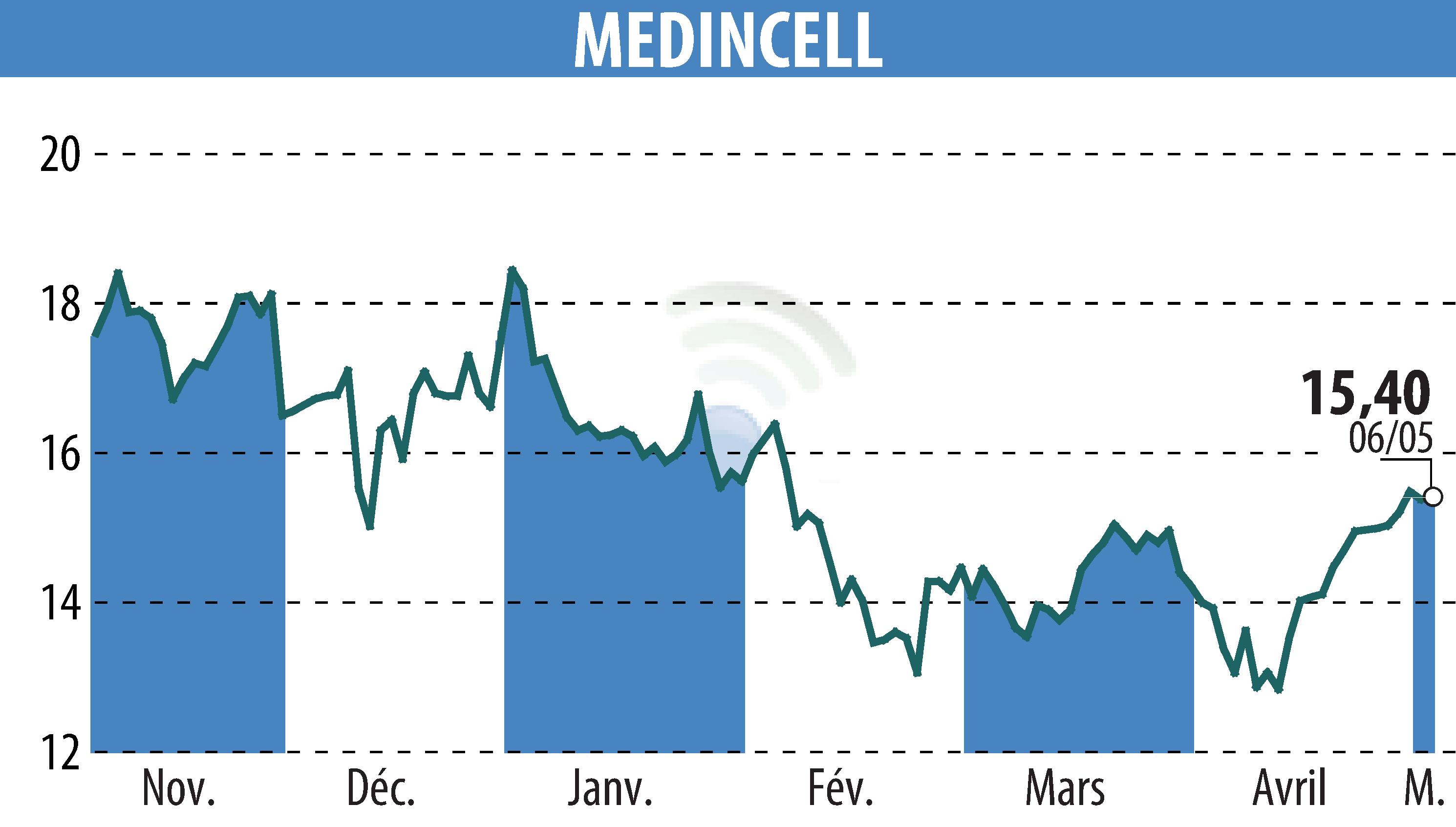
sur MEDINCELL (EPA:MEDCL)
Medincell's Growth in Antipsychotic Treatments
Medincell reports the ongoing success of UZEDY®, a subcutaneous risperidone treatment for schizophrenia. Since its U.S. launch in May 2023, UZEDY® has seen increasing demand. In Q1 2025, sales reached $39 million, a notable rise compared to the previous year. Medincell benefits from this partnership with Teva Pharmaceuticals through royalties and potential commercial milestones amounting to $105 million.
Furthermore, Medincell is progressing with its Olanzapine Long-Acting Injectable (LAI) project. The pivotal Phase 3 trial concluded in January 2025, showing positive results without post-injection complications. The company plans to submit a New Drug Application (NDA) in the second half of 2025, following a productive meeting with the FDA in April 2025.
Medincell is positioning itself strongly in the antipsychotic market, aiming to deliver safer and accessible treatments for schizophrenia through its innovative drug delivery technologies.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de MEDINCELL
