sur Nanohale AG (ETR:FYB)
Formycon AG Updates on Biosimilar Projects and Strategic Adjustments
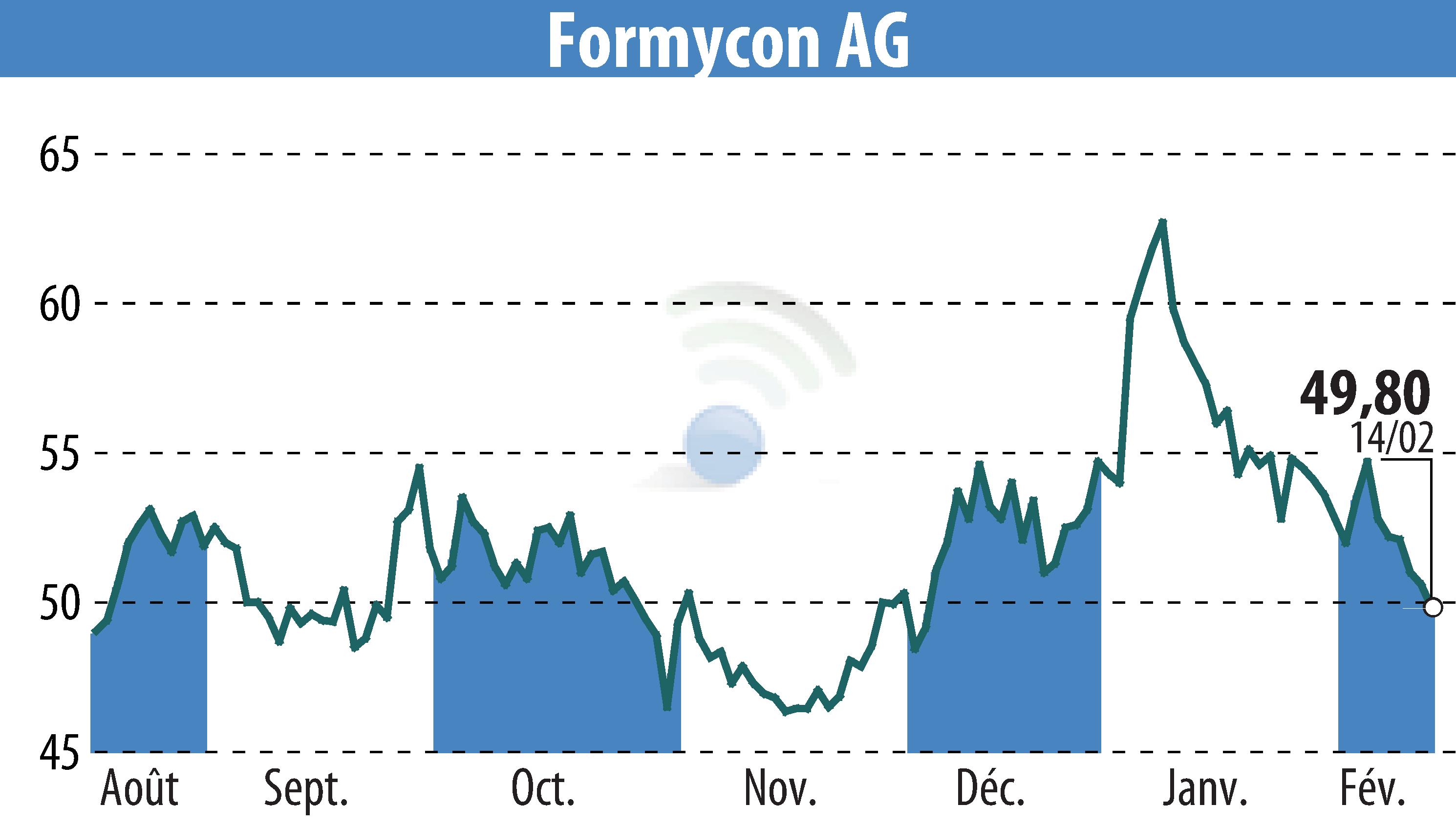
Formycon AG announced key updates on its biosimilar projects, highlighting financial and strategic adjustments. The company decided to halt the Phase III trial for FYB206, a pembrolizumab biosimilar, potentially saving a significant double-digit million amount. This move is based on successful discussions with the FDA, indicating that existing analytical and Phase I trial data are sufficient for U.S. approval.
Revisions are also anticipated in the valuation of FYB202/Otulfi™ due to expected higher price discounts in the U.S., potentially leading to a non-cash impairment. Meanwhile, discussions are underway with Sandoz AG for FYB201/CIMERLI® to address commercialization challenges driven by increased price discounts, which might result in a temporary pause in U.S. commercialization.
Overall, these developments should not impact 2024's financial guidance, although net results may be affected due to impairments. Formycon continues to focus on achieving medium-term profitability and positive cash flow.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Nanohale AG
