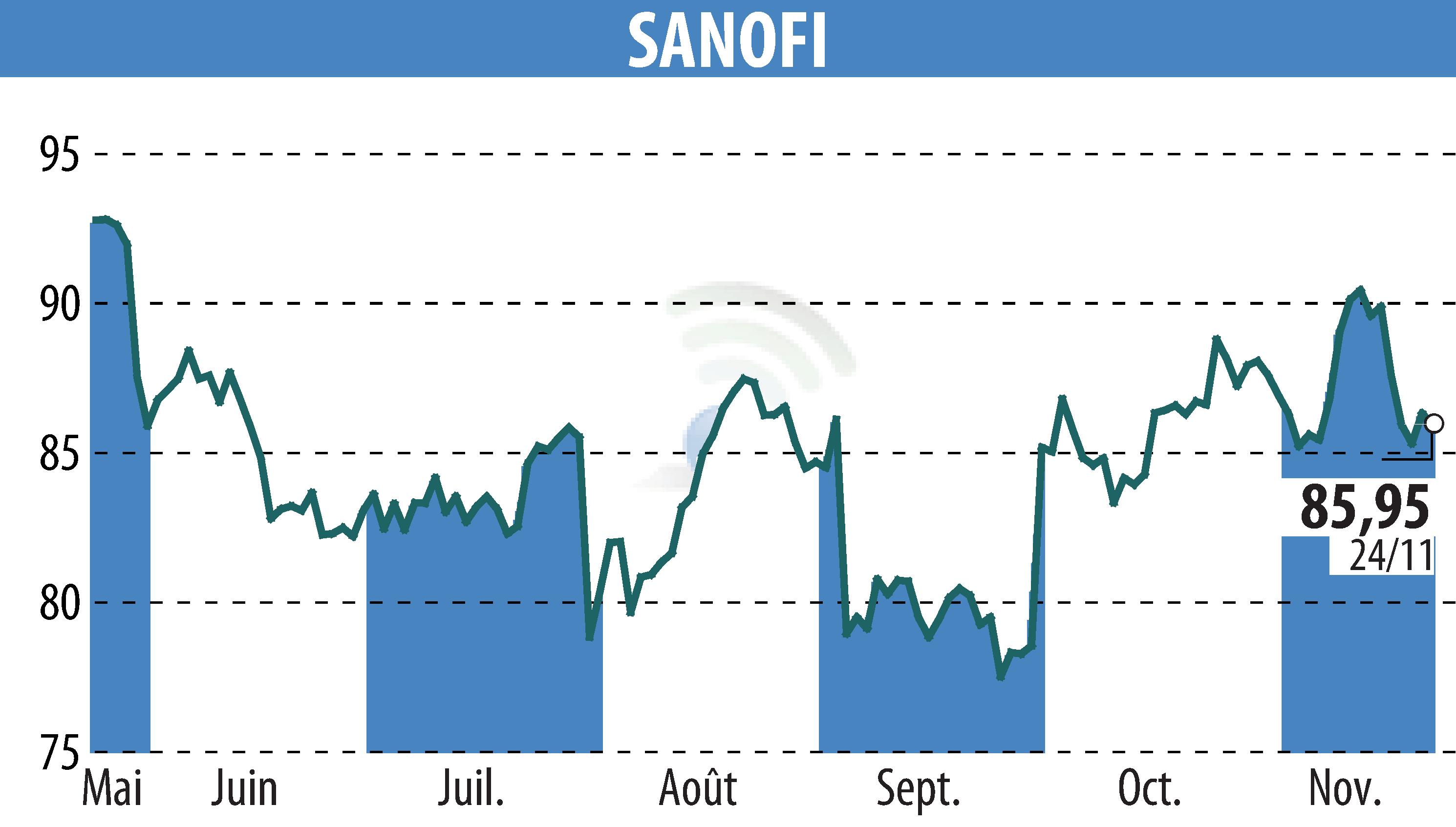
sur SANOFI-AVENTIS (EPA:SAN)
Dupixent: a breakthrough in the treatment of chronic spontaneous urticaria in Europe
The European Commission has approved Dupixent, co-developed by Sanofi and Regeneron, as a treatment for moderate to severe chronic spontaneous urticaria (CSU) in adults and adolescents aged 12 years and older. It is the first targeted therapy approved in the EU for this condition in over ten years.
Based on phase 3 studies, the approval demonstrates a significant reduction in itching and hives over 24 weeks compared to placebo. Dupixent inhibits IL4 and IL13, key to type 2 inflammation, and is already used to treat seven chronic inflammatory diseases.
Approximately 270,000 people in the EU suffer from chronic obstructive pulmonary disease (COPD), often without an adequate response to standard antihistamines. Dupixent therefore offers a new option to improve the quality of life for these patients.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
