sur SANOFI-AVENTIS (EPA:SAN)
Sanofi Faces Delay in FDA Approval for Tolebrutinib in MS Treatment
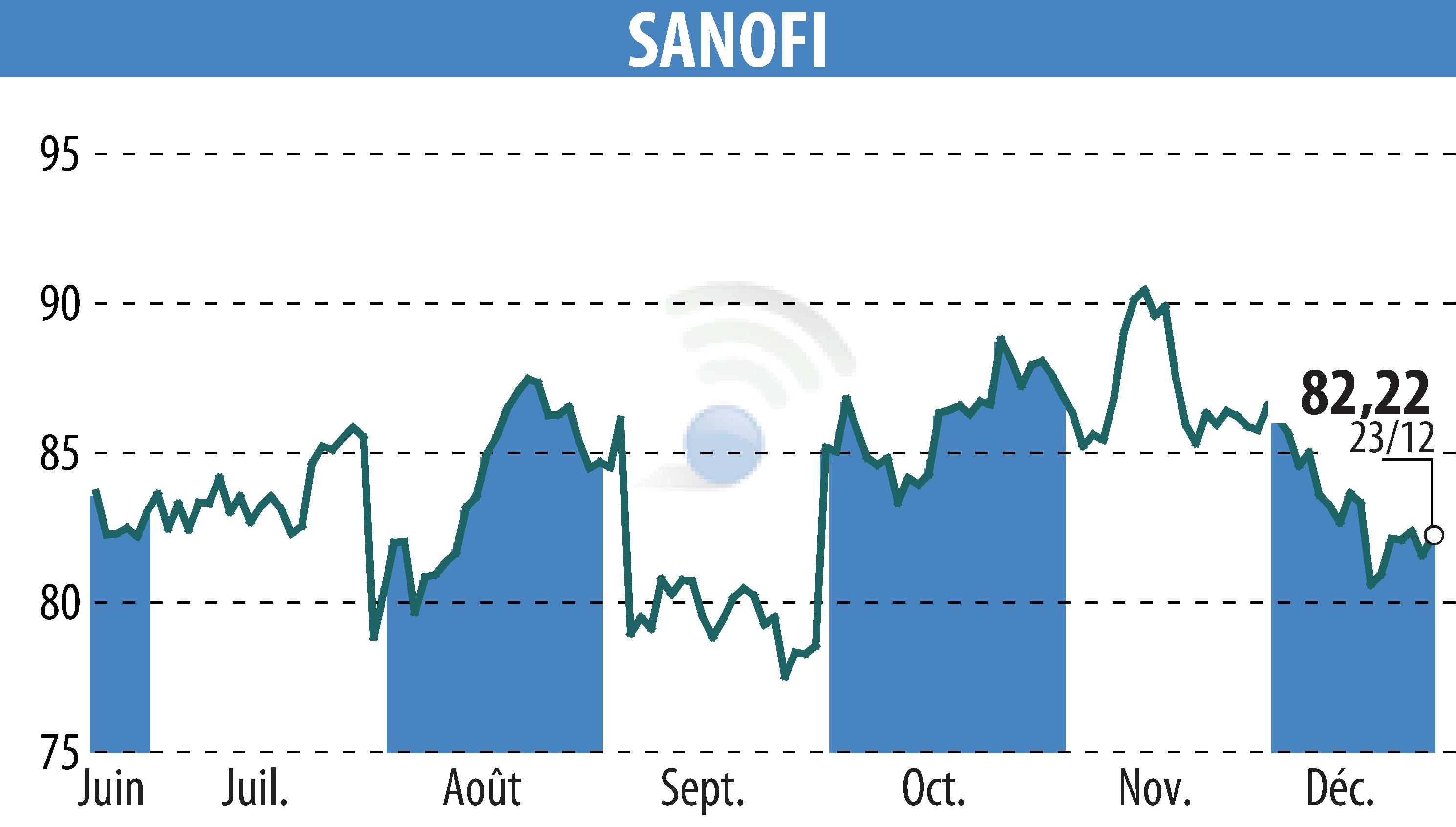
Sanofi has announced a delay in its regulatory submission for tolebrutinib, aimed at treating non-relapsing secondary progressive multiple sclerosis (nrSPMS) in adults. The US FDA issued a complete response letter for the new drug application, leading to an extended review beyond the initially set target date of December 28, 2025. Sanofi anticipates further guidance from the FDA by the end of the first quarter of 2026.
Despite the setback, Sanofi remains involved, having submitted an expanded access protocol for the drug following FDA's request. Tolebrutinib was already provisionally approved in the UAE and is under regulatory review in the EU and other regions. The company is conducting an impairment test on the intangible asset value attached to tolebrutinib, which will not affect 2025's financial guidance.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
