sur SANOFI-AVENTIS (EPA:SAN)
Sanofi: PERSEUS study results on tolebrutinib disappointing
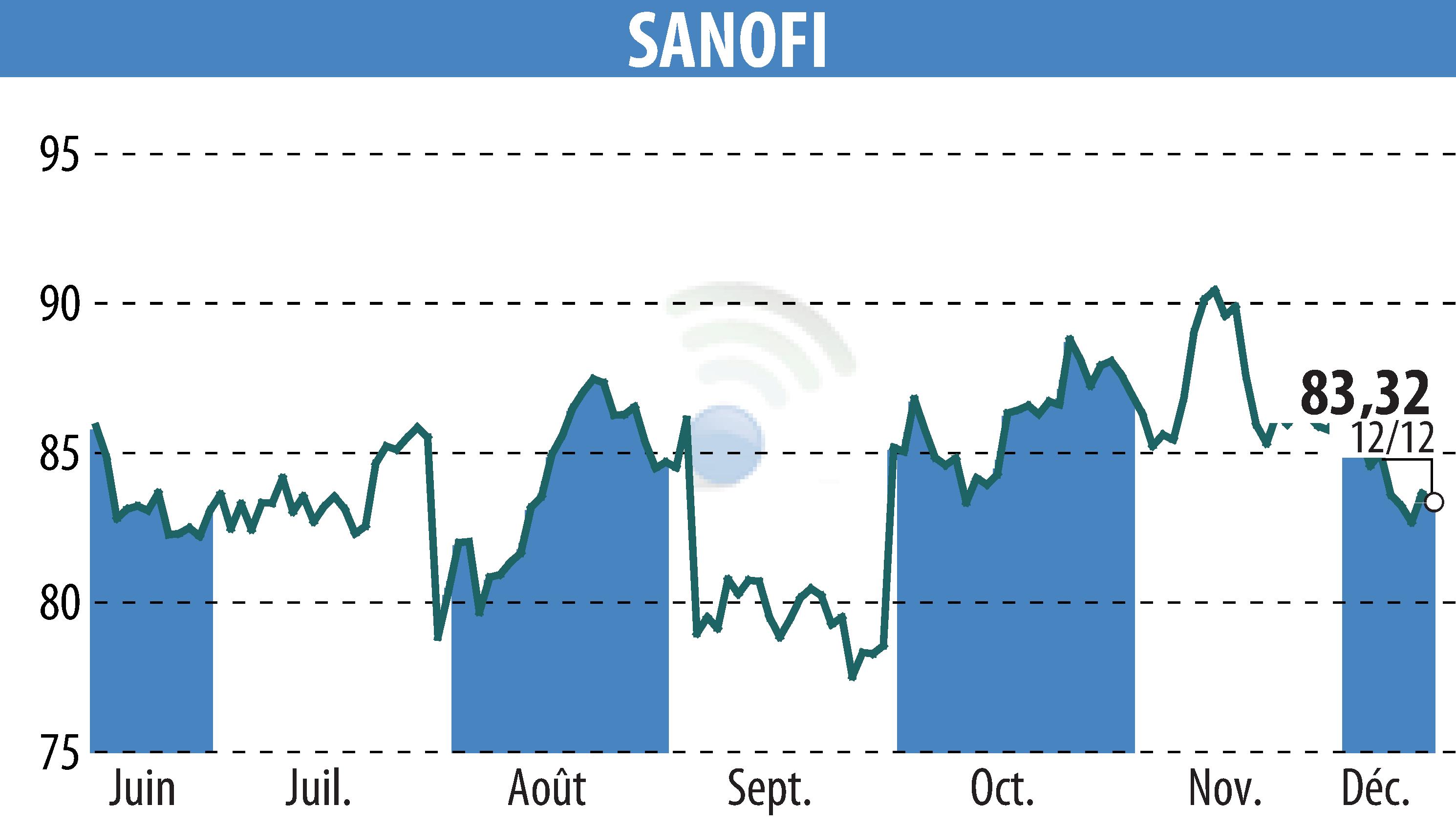
Sanofi has announced the results of its Phase 3 PERSEUS study of tolebrutinib, a treatment targeting primary progressive multiple sclerosis (PPMS). The study did not meet its primary endpoint of delaying disability progression compared to placebo. These results mean that Sanofi will not seek regulatory approval for PPMS.
The safety profile of tolebrutinib remained consistent with previous studies, despite identified liver risks. Detailed results will be presented at a future medical conference. In July 2025, tolebrutinib received temporary approval in the United Arab Emirates for another type of multiple sclerosis.
Sanofi will conduct an impairment test on the intangible asset related to tolebrutinib, with no impact on 2025 financial results. The company continues to seek solutions to unmet medical needs in multiple sclerosis.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
