sur SANOFI-AVENTIS (EPA:SAN)
Sanofi's rilzabrutinib granted orphan drug designation in the EU
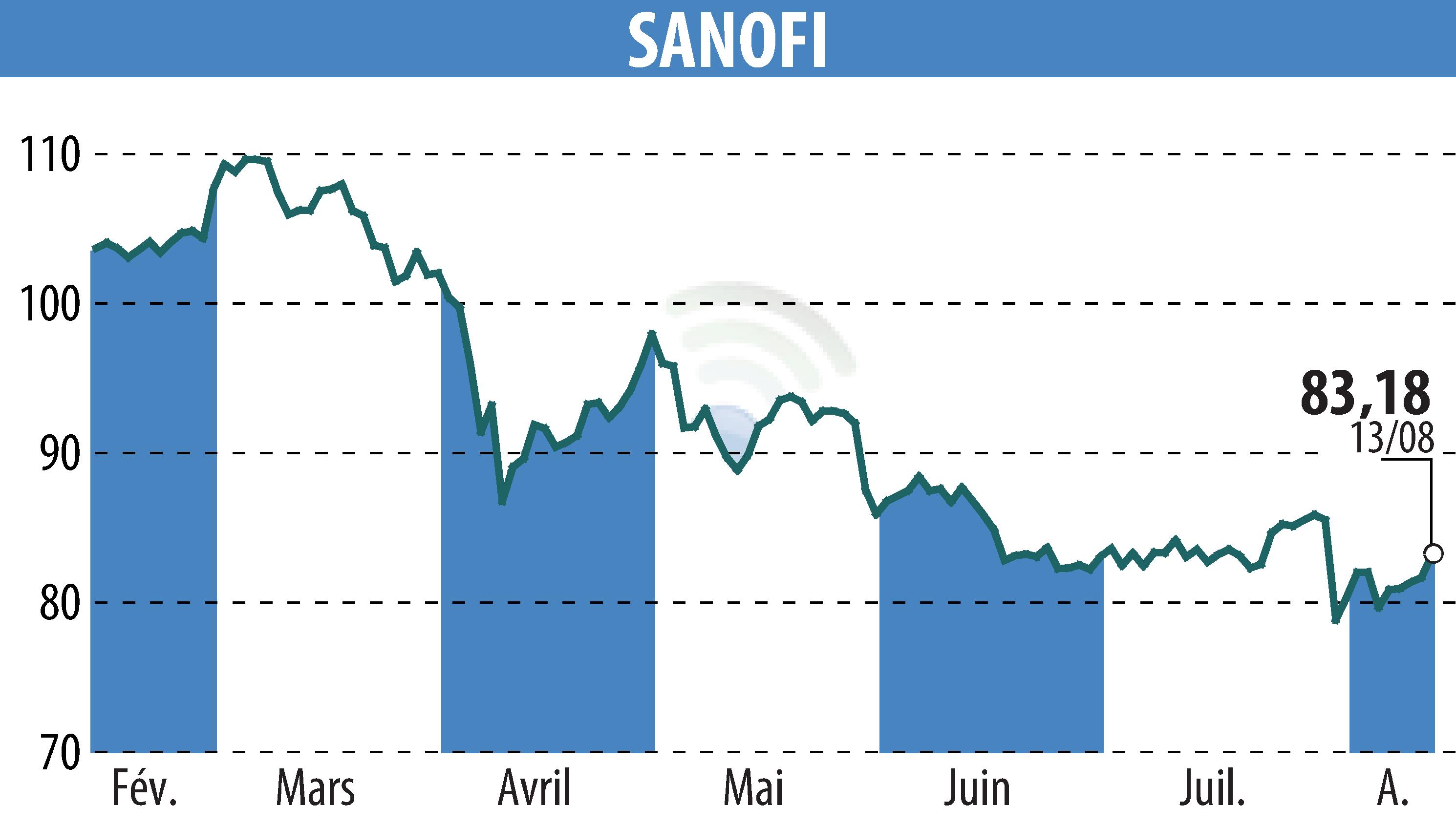
On August 14, 2025, the European Medicines Agency granted orphan drug designation to rilzabrutinib, a Bruton's tyrosine kinase inhibitor. This investigational treatment targets IgG4-mediated disease. This recognition underscores Sanofi's commitment to rare immune-mediated diseases. Results from the Phase 2 study showed a reduction in symptoms in patients treated for 52 weeks.
In addition to this designation, rilzabrutinib has also been granted orphan drug status for other diseases such as immune thrombocytopenia in the EU, the US, and Japan. In the US, it also has Fast Track designation. Sanofi is currently awaiting the FDA's decision on its potential use in immune thrombocytopenia.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
