sur SENSORION (EPA:ALSEN)
Sensorion Completes Enrollment for Audiogene Phase 1/2 Trial
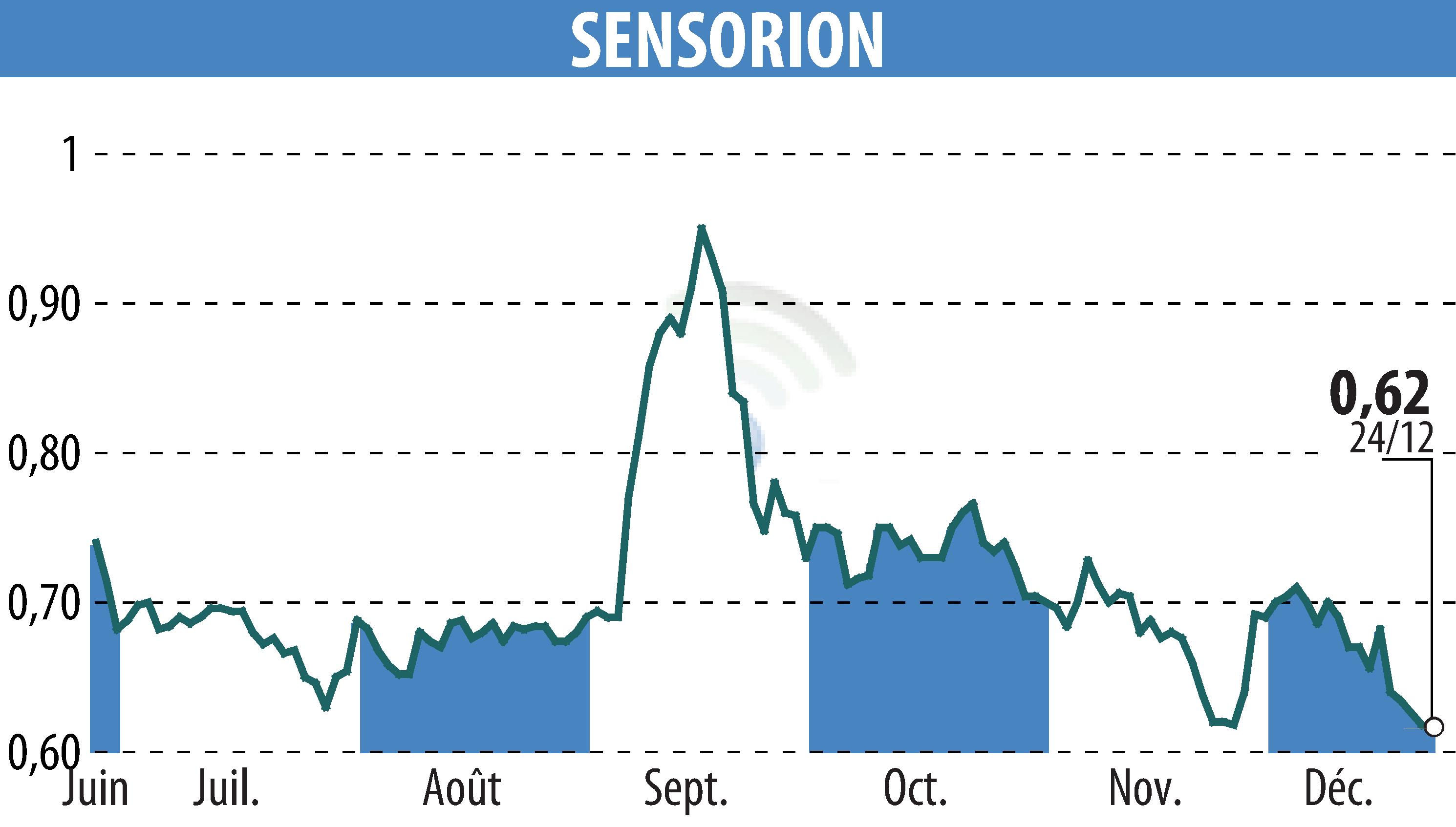
On December 27, 2024, Sensorion announced the successful completion of patient enrollment in the first cohort of its Audiogene Phase 1/2 clinical trial. This trial involves SENS-501, a gene therapy aimed at treating hearing loss due to otoferlin gene mutations. The trial targets infants and toddlers aged 6 to 31 months.
The first cohort has three patients, with the last patient receiving the SENS-501 injection in December 2024. The surgical administration was tolerated without serious adverse events. The trial's goal is to measure SENS-501's capacity to restore hearing and facilitate normal speech development in these young patients.
Sensorion's CEO, Nawal Ouzren, emphasized the trial's progress as a major achievement and anticipates the 2025 KOL event where additional data will be presented and analyzed.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SENSORION
