sur SENSORION (EPA:ALSEN)
Sensorion Reports Positive Preliminary Data from Audiogene Phase 1/2 Trial
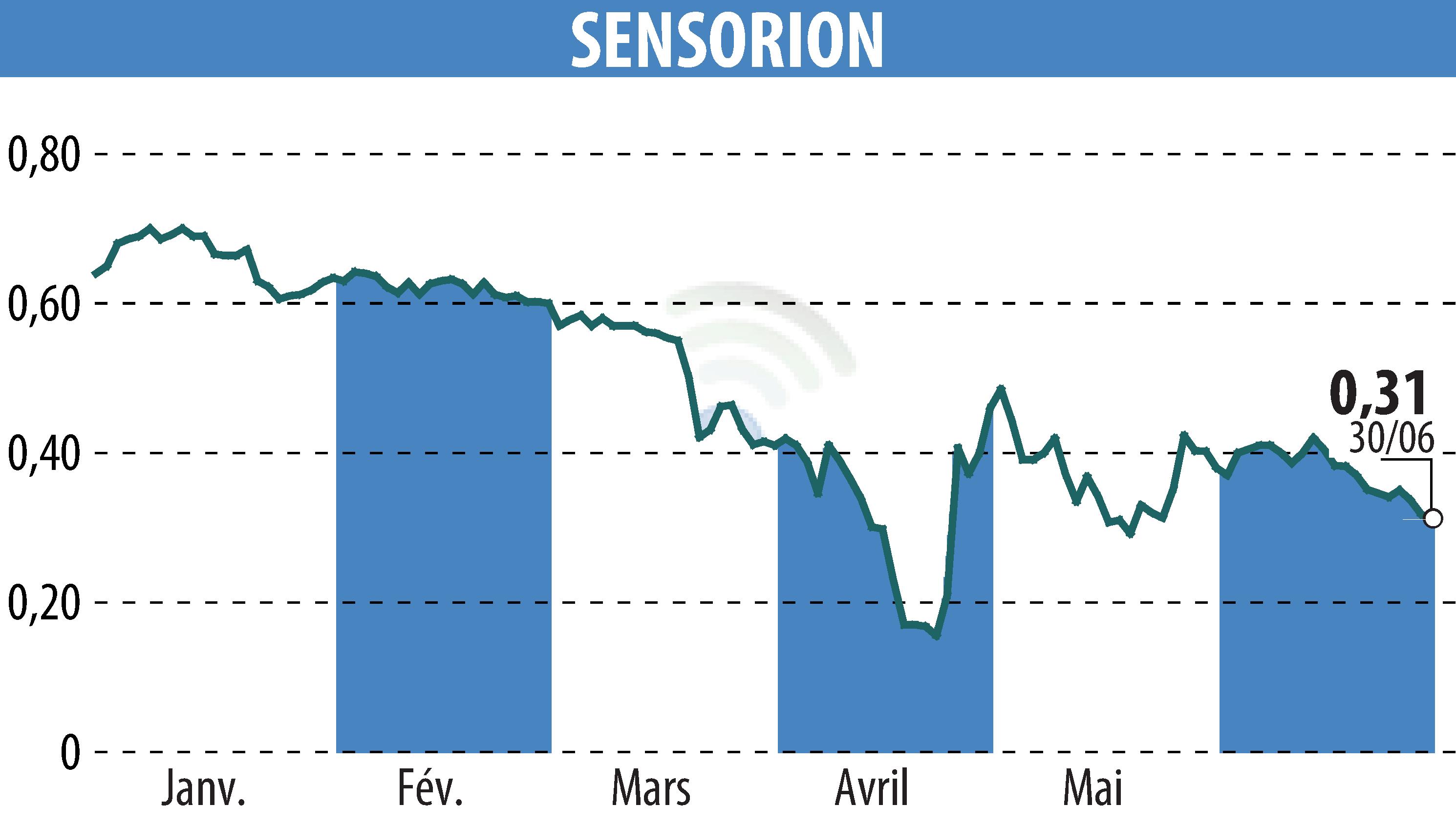
Sensorion has announced positive preliminary results from the first cohort of its Audiogene Phase 1/2 gene therapy clinical trial. The trial evaluates SENS-501, a gene therapy candidate intended to treat congenital deafness linked to OTOF gene mutations. All five patients treated so far showed good tolerability to the therapy and surgical delivery, with no serious adverse events.
In cohort one, early signs of hearing improvement were observed in an 11-month-old toddler, with significant ABR and PTA test results and notable changes in auditory responses reported by parents. Recruitment for a second cohort, involving a higher dose, is nearly complete.
The trial aims to determine the safety and efficacy of intra-cochlear SENS-501 injections in pediatric patients aged 6 to 31 months. Sensorion plans to provide updates as cohort data matures.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SENSORION
