sur THERACLION (EPA:ALTHE)
Theraclion Submits Sonovein to FDA After Year of Key Achievements
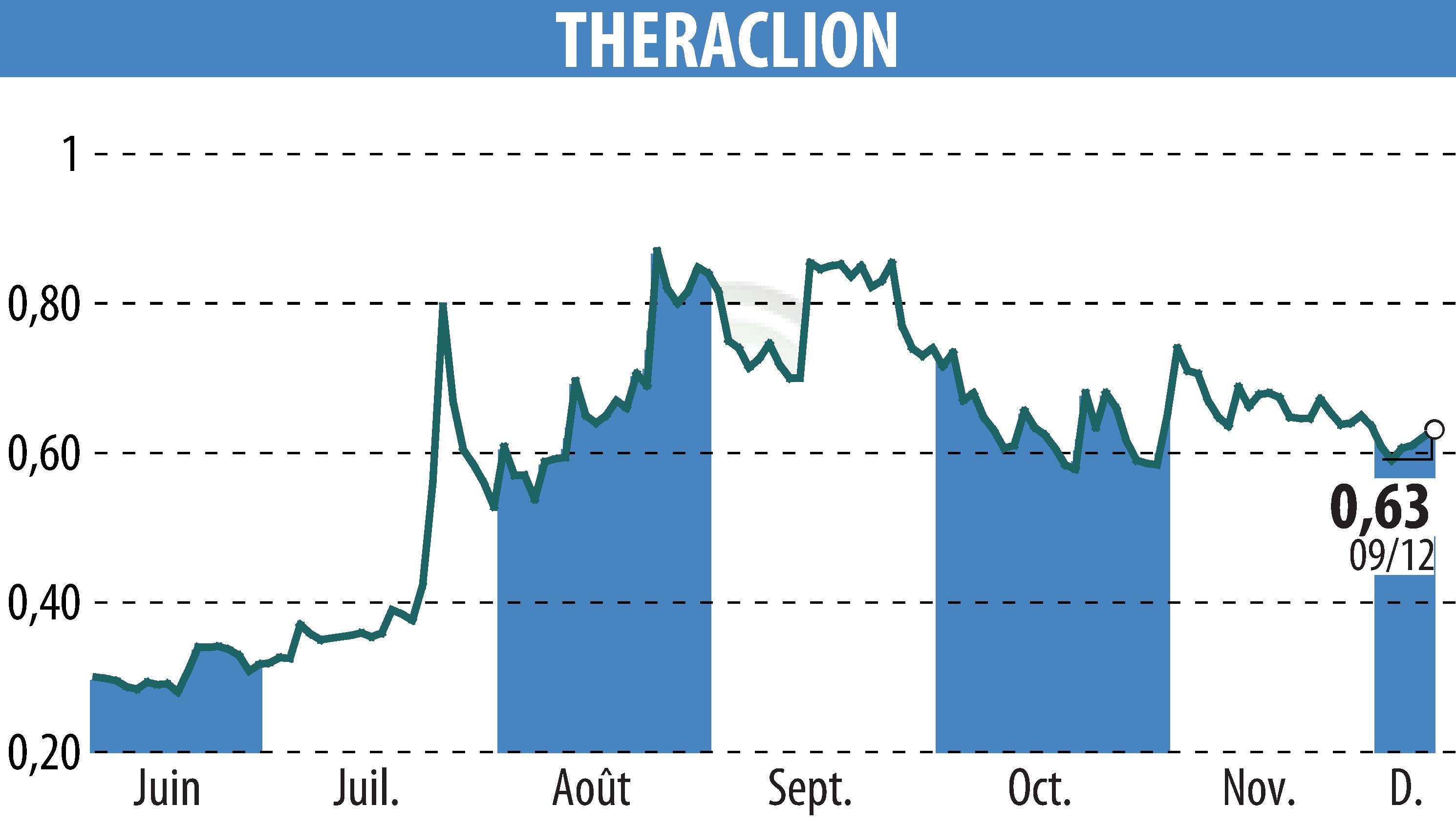
Theraclion, a company focusing on non-invasive varicose vein treatments, has announced the submission of its Sonovein® platform to the FDA. This pivotal move marks the culmination of a year filled with significant regulatory and clinical progress. The data packet submission is crucial for De Novo clearance, potentially opening doors to the extensive U.S. market by mid-2026.
Beyond the U.S., Sonovein® has gained important regulatory approvals in Europe and China, achieving EU MDR certification and compliance with China's standards. This progress reinforces its potential for future commercialization in these regions.
Clinical evidence reinforcing Sonovein®'s efficacy was strengthened through various peer-reviewed studies in 2025, with impressive results in terms of vein occlusion and patient safety. The year also saw active participation in 16 international congresses, enhancing visibility among medical professionals globally.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de THERACLION
