sur THERACLION (EPA:ALTHE)
Theraclion submits Sonovein to the FDA after significant progress
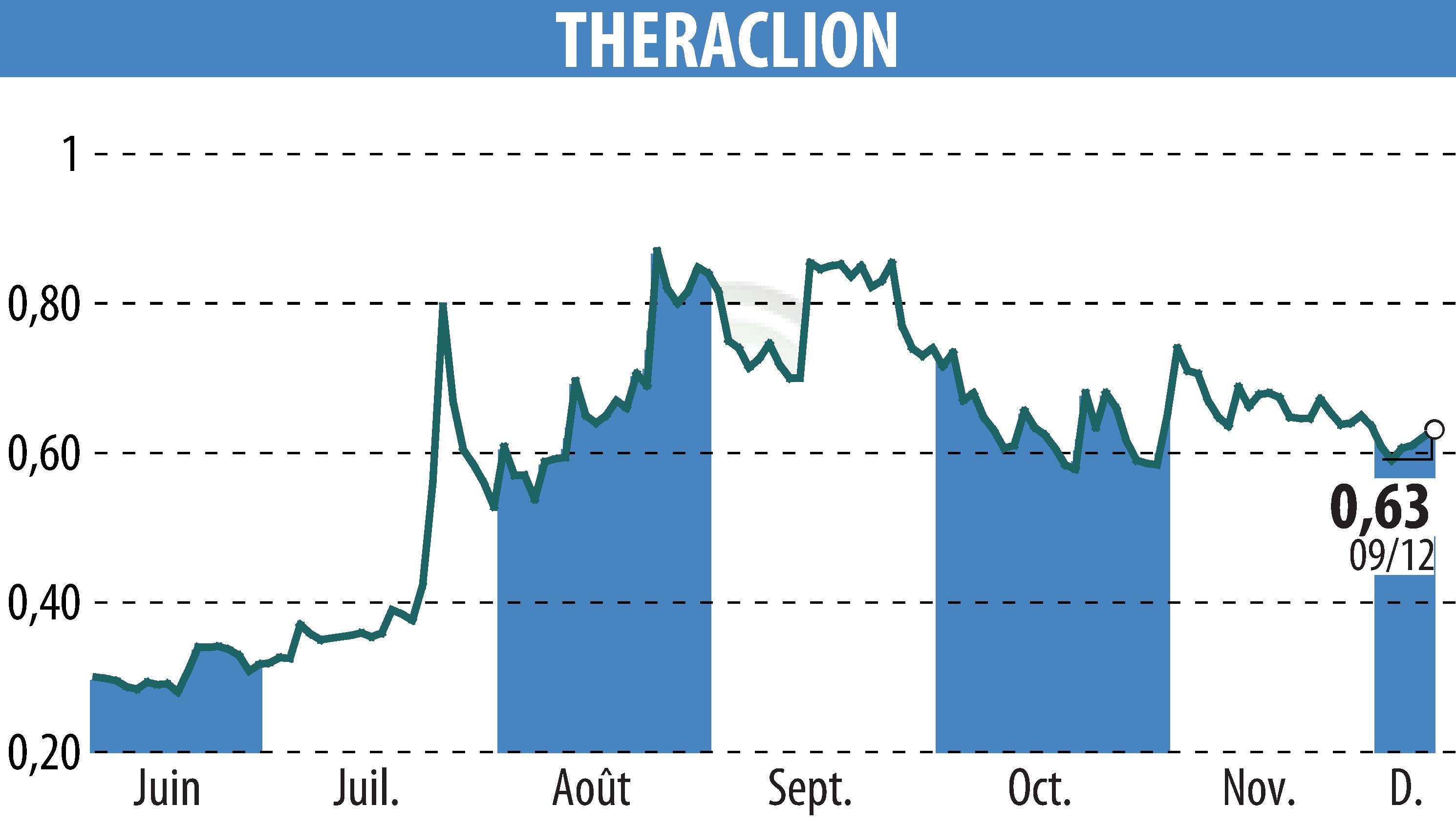
On December 9, 2025, Theraclion announced the submission of its Sonovein® device to the FDA. A decision on this De Novo clearance application could be reached by mid-2026. Approval would allow Sonovein® to enter the US market, the largest for varicose vein treatment.
In 2025, Sonovein® received MDR certification in Europe and met the Chinese standard GB 9706.1-2020. Scientific publications have confirmed its effectiveness, including studies showing up to 97% effectiveness at 12 months.
Theraclion's presence at 16 congresses has increased its visibility among specialists. The value proposition of Sonovein®, combined with its effectiveness, was well received.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de THERACLION
