sur VALNEVA (EPA:VLA)
FDA suspends IXCHIQ® license in the United States
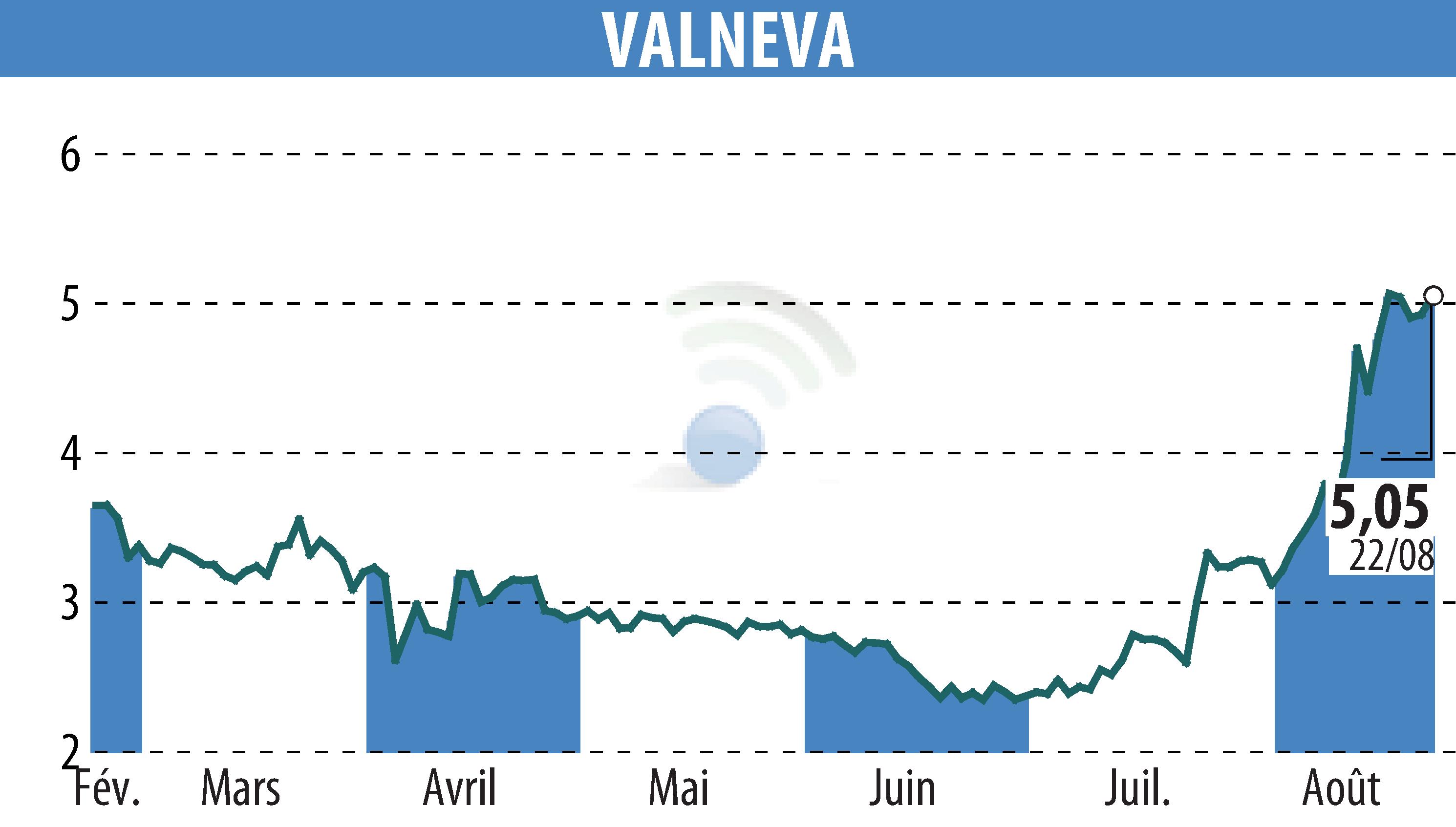
On August 25, 2025, Valneva SE announced that the Food and Drug Administration (FDA) has suspended the U.S. license for IXCHIQ®, its chikungunya vaccine. This decision comes after the reporting of four new cases of serious adverse events, suggestive of the disease itself. Three of these cases involved people aged 70 to 82.
This suspension follows an earlier partial lifting of the suspension, targeting people aged 60 and over. Valneva emphasizes that the reported incidents are not unprecedented, having already been observed during clinical trials. The company is committed to following the highest safety standards and actively collaborating with health authorities.
Valneva is currently assessing the potential financial impact of this suspension. Despite this uncertainty, the company is not changing its revenue guidance, with IXCHIQ® representing a minor portion of its revenue in the first half of 2025.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de VALNEVA
