sur VALNEVA (EPA:VLA)
Valneva's Lyme Disease Vaccine Makes Significant Progress
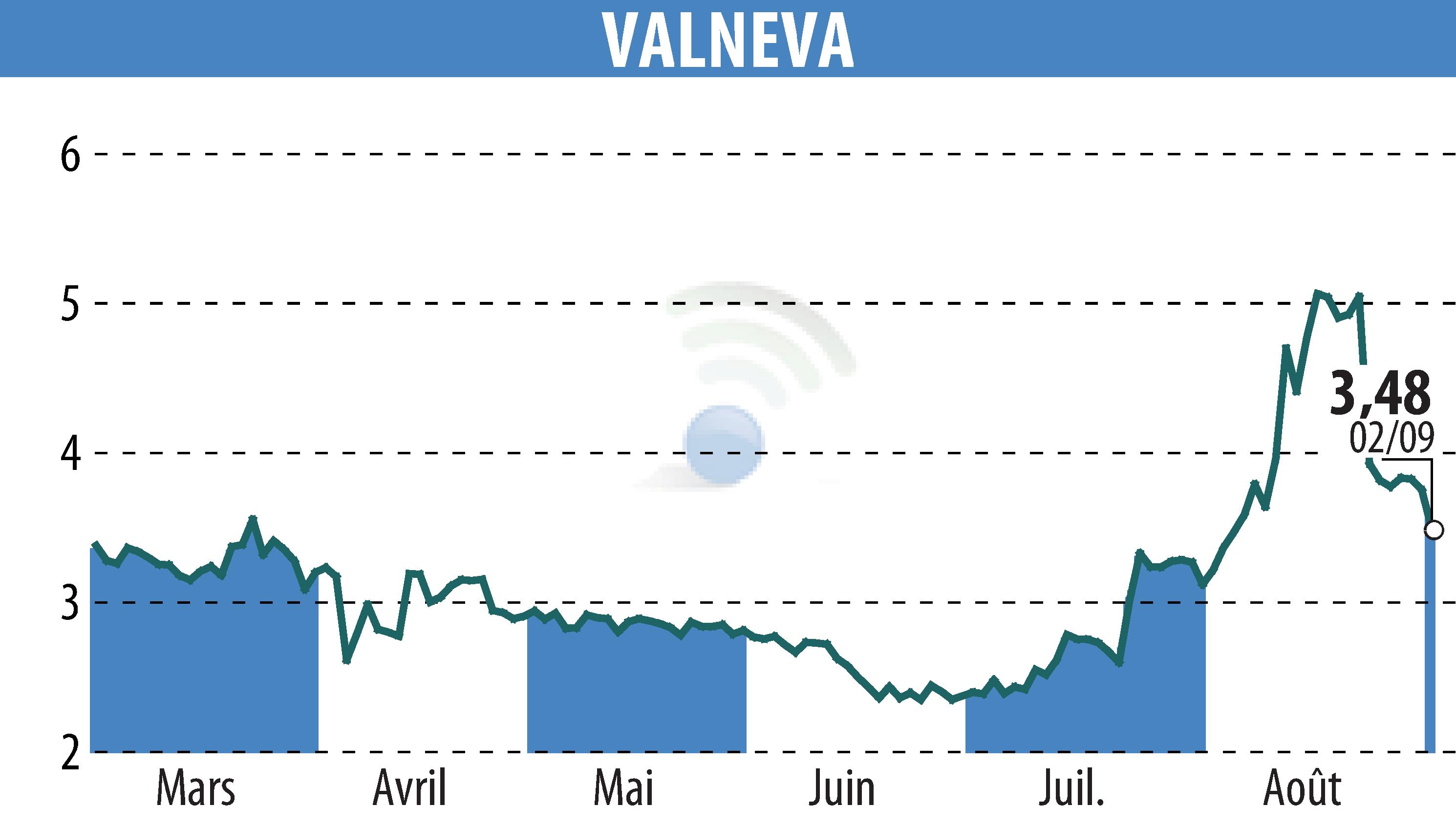
On September 3, 2025, Valneva SE announced promising Phase 2 results for its Lyme disease vaccine candidate, VLA15. The data show a strong immune response after a third booster dose in children and adults, with a notable anamnestic response for all six targeted serotypes.
No safety issues have been reported, corroborating previous findings. Currently, there is no human vaccine against this disease, which is spreading rapidly. The two ongoing Phase 3 trials could lead Pfizer to submit marketing authorization applications in 2026.
The Phase 3 study is examining the efficacy and safety of VLA15, which is being administered in endemic areas of North America and Europe. The development of this vaccine, in collaboration with Pfizer, addresses a growing medical need.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de VALNEVA
